Chapter 45 Kawasaki Disease
Kawasaki disease, initially described by Dr. Tomisaku Kawasaki in 1967,1 is an acute systemic vasculitis of uncertain etiology that predominantly affects infants and young children. The disease has been described worldwide and occurs in all populations. The classic presentation of the illness is marked by at least 4 days of fever, oral mucositis, nonexudative conjunctivitis, erythematous nonvesicular rash, changes in the hands and feet including edema or erythema, and cervical lymphadenopathy2 (Box 45-1). Coronary artery dilation or aneurysms affect up to 25% of those who are not treated with intravenous gamma globulin (IVIG) early in the course of the disease. In patients who develop aneurysms, angina, myocardial infarction (MI), and death may ensue during the acute phase3 or months to years later.4
![]() Box 45-1 Principal Clinical Findings in Kawasaki Disease*
Box 45-1 Principal Clinical Findings in Kawasaki Disease*
Fever persisting at least 4 days or more without other source in association with at least 4 principal features:
Adapted from American Heart Association Scientific Statement: Diagnosis, treatment, and long-term management of Kawasaki disease. Circulation 110:2751, 2004.
Epidemiology
Despite its description in diverse areas of the world, by far the greatest number of cases have been reported in Japan. Indeed, in the most recent Japanese nationwide survey performed in calendar years 2007 and 2008, the incidence rate was over 200 per 100,000 children younger than 5 years of age.5 In Japan, 1.4% of cases occur in children with a previously affected sibling. The disease can recur (≈︀3.5% in Japan), but person-to-person transmission is unusual. Lack of a mandatory national reporting system in the United States hinders epidemiological analysis, but administrative data from hospital discharge abstracts suggested that more than 4000 U.S. hospitalizations were associated with Kawasaki disease in the year 2000.6 Among children younger than age 5, occurrence was greatest in Asians (33.3/100,000), somewhat less in African Americans (23.4/100,000), and lowest in Caucasians (12.7/100,000).6 Outbreaks are more likely in the late winter and early spring, suggesting an infectious etiology, but a steady background activity of cases is noted throughout the remainder of the year. Males outnumber females, generally in a ratio of 1.4:1. Although Kawasaki disease is most common in children younger than age 5, the illness is being more commonly recognized in older children and adolescents.7
Etiology and Pathogenesis
The search for an etiological agent has been wide-ranging over the course of the past 3 decades, but to date the cause of Kawasaki disease is unknown. Although Kawasaki disease is not spread by person-to-person transmission, features suggesting an infectious etiology include its peak incidence in young children; clinical features including fever, rash, and conjunctivitis; the increase in incidence in winter and early spring; and the past occurrence of nationwide epidemics in Japan. Prior exposure of index cases to freshly cleaned carpets has been reported to be a risk factor by some investigators,8 although other studies have not confirmed this association,9 and no specific organism or toxin has been identified in the rugs. Some researchers hold that Kawasaki disease is caused by a single agent,10 but others believe the marked immune response typical in Kawasaki disease can be triggered by a variety of different agents.11 Reports of selective expansion of Vβ2 and Vβ8 T-cell receptor families have implicated specific superantigens, including TSST-1-secreting strains of Staphylococcus aureus and streptococcal pyrogenic exotoxin B- and C-producing streptococci.12 The only animal model of Kawasaki disease, which uses Lactobacillus casei to induce coronary arteritis in mice, implicates a toxin-mediated etiology.13 By contrast, Rowley has found support for a typical antigen-driven response by demonstrating oligoclonal immunoglobulin (Ig)A plasma cells and IgA heavy chain genes, as well as macrophages and CD8 T lymphocytes in inflamed arterial tissue of individuals with fatal Kawasaki disease.14 With a synthetic IgA antibody, these investigators have demonstrated intracytoplasmic inclusion bodies with aggregates of nucleic acids and viral proteins in the proximal bronchial epithelium and coronary arteries in most postmortem specimens from Kawasaki disease.15 These ribonucleic acid (RNA)-containing cytoplasmic inclusion bodies were demonstrated in 85% of postmortem specimens from Kawasaki disease patients but few young age-matched children. The finding that 25% of older children but adult controls have such inclusion bodies postmortem is consistent with the hypothesis that Kawasaki disease could be caused by a ubiquitous RNA virus that persists and causes the clinical features of Kawasaki disease in susceptible children.16,17
Kawasaki disease is accompanied by significant derangements in the immunoregulatory system that lead to coronary inflammation and coronary artery abnormalities (dilation, aneurysm formation, and giant aneurysms) in some patients. Profound immune activation is evidenced by release of proinflammatory cytokines and growth factors, endothelial cell (EC) activation, and infiltration of the coronary arterial wall first by neutrophils, and then by CD68+ monocyte/macrophages, CD8+, CD3+, and CD20+ lymphocytes, and IgA plasma cells.18–26
Release of matrix metalloproteinases (MMPs) may further disrupt arterial wall integrity, leading to aneurysms of the coronary arteries and occasionally of other extraparenchymal medium-sized muscular arteries.27 Kawasaki disease patients with coronary artery lesions have higher levels of MMPs and higher ratios of MMPs to tissue inhibitors of metalloproteinases (TIMPs) than those without coronary abnormalities,28 suggesting these circulating proteins may play an active role in coronary arterial remodeling. Data to suggest an important role for MMPs in the pathogenesis of aneurysms also comes from recent research on genetic risk factors, showing that MMP-3 rs3025058 (−/T) and haplotypes containing MMP-3 rs3025058 (−/T) and MMP-12 rs2276109 (A/G) were associated with a higher risk of aneurysm formation.29
Genetic factors have long been recognized to play an important role in susceptibility to Kawasaki disease. Children of Japanese ancestry have a relative risk 10 to 15 times higher than that of Caucasian children, whether they live in Japan or the United States. Furthermore, siblings have a relative risk 6 to 10 times greater than that of children without a family history. The parents of Japanese children with Kawasaki disease are twice as likely to have had the disease themselves in childhood than expected in the general population.30 In addition to MMP haplotypes, increased susceptibility to Kawasaki disease has been related to genetic variations in the transforming growth factor (TGF)-β pathway,31 CC chemokine receptor 5 (CCR5) and/or its ligand CCL3L1,32 and ITPKC (a negative regulator of T cell activation)33 among others. In a genomewide association study, investigators recently identified a single functional network containing LNX1, CAMK2D, ZFHX3, CSMD1, and TCP1, believed to be relevant to inflammation and apoptosis.34 Functional genomics may eventually allow development of new diagnostic tests and therapies for Kawasaki disease.
Pathology
Because mortality in this disease is uncommon (ranging from 0%-0.17% in the United States), published studies on the histopathology of early Kawasaki disease are limited.35,36 The original pathological description of Kawasaki disease by Fujiwara and Hamashima was based on autopsy findings of children dying in the acute and subacute phases of Kawasaki disease before available treatment with IVIG.35 In this early study, they established four categories of illness on the basis of time from onset of the disease: stage 1 (0-9 days), stage 2 (12-25 days), stage 3 (28-31 days), and stage 4 (40 days-4 years). Stage 1 was characterized by vasculitis of small vessels and microvessels, perivasculitis and endarteritis of the coronary vessels, and pancarditis. Pancarditis was seen also in stage 2, with coronary artery vasculitis, sometimes with aneurysm formation and coronary thrombosis. By stage 3, the acute inflammation had subsided, but myointimal proliferation of the coronary arteries was evident. In stage 4, coronary artery stenosis was noted for the first time. In stage 1, death resulted from arrhythmia and myocarditis, with evolution to ischemia and infarction with greater time from onset of disease to postmortem examination. Indeed, patients who die late after Kawasaki disease often have coronary artery stenoses resulting from neointimal proliferation and fibrosis.35,36 Within aneurysms, the internal elastic lamina is disrupted, and medial smooth muscle is replaced by fibroblasts and extracellular matrix (ECM).37 Finally, growth factors are expressed in the areas subjected to the greatest shear stress, particularly at the proximal and distal ends of an aneurysm.38 Peripheral artery aneurysms (e.g., occurring in axillary or iliac arteries) are found only among patients with giant coronary artery aneurysms.
Clinical Presentation
Because no laboratory test or pathognomonic feature is available for Kawasaki disease, the diagnosis must be made on clinical grounds. First described by Kawasaki on the basis of his observations in Japanese children,39 the classic criteria have continued to serve as the standard adopted by the American Heart Association (AHA; see Box 45-1) for arriving at the diagnosis.2 They include fever for 4 days or more and at least 4 of the 5 following findings: (1) a nonexudative bilateral conjunctivitis, (2) oral changes with erythematous or dry cracked lips, strawberry tongue, or pharyngitis, (3) a nonvesicular rash, often involving the trunk, perineum, and extremities, (4) erythema of the palmar and plantar surfaces, edema of the hands or feet, or periungual desquamation 2 weeks after illness onset, and (5) anterior cervical lymphadenopathy of 1.5 cm or greater, usually unilateral. Alternatively, the diagnosis can be made with fewer than 4 of 5 criteria in the presence of coronary artery abnormalities. Not all criteria have to be present simultaneously to make the diagnosis; indeed, it is common for some findings to resolve as others appear, making serial evaluation of the child essential.
The epidemiological case definition is not fulfilled in almost a third of children who develop coronary artery aneurysms.40 To capture incomplete cases, the 2004 AHA recommendations include an algorithm for evaluation and treatment of suspected Kawasaki disease in children with at least 5 days of fever and only two or three clinical criteria.2 Furthermore, infants younger than 6 months of age present a particular challenge because they often have incomplete criteria, yet are at greater risk for development of coronary artery abnormalities. The diagnosis should be considered and echocardiography performed in young infants who have fever for at least 7 days without documented source and whose laboratory tests indicate substantial systemic inflammation.2
Other supportive signs are present in many children with Kawasaki disease (Box 45-2). The rash, when perineal in location, often desquamates by the end of the first week of illness. Anterior uveitis can be identified by slit-lamp examination in 83% of patients early in the course.41 Arthralgia and arthritis of large and small joints may be severe enough that children refuse to walk or perform tasks with their hands, but the arthritis is virtually never chronic. Abdominal signs including vomiting, diarrhea, or hydrops of the gallbladder are common.
![]() Box 45-2 Other Significant Clinical and Laboratory Findings in Kawasaki Disease
Box 45-2 Other Significant Clinical and Laboratory Findings in Kawasaki Disease
Cardiovascular
On auscultation, gallop rhythm or distant heart sounds; ECG changes (arrhythmias, abnormal Q waves, prolonged PR or QT intervals or both; occasionally low-voltage or ST-T wave changes); chest x-ray abnormalities (cardiomegaly); echocardiographic changes (pericardial effusion, coronary aneurysms, or decreased contractility); mitral or aortic valvular insufficiency or both; and (rarely) aneurysms of peripheral arteries (e.g., axillary), angina pectoris, or MI
Adapted from American Heart Association Scientific Statement: Diagnosis, treatment, and long-term management of Kawasaki disease. Circulation 110:2751, 2004.
Laboratory values in the acute phase are consistent with systemic inflammation. Acute-phase reactants, including erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), are markedly increased. White blood cell (WBC) count is elevated with a leftward shift, and a normochromic, normocytic anemia is noted within the first week of illness. Thrombocytosis is usually present by the second week of the disease, often peaking at counts greater than 1,000,000 mm3 in association with hypercoagulability. These parameters often persist over the first month of the illness and gradually decline. Hepatocellular inflammation is accompanied by increases in γ-glutamyl transferase. Sterile pyuria and pleocytosis of cerebrospinal fluid,42 both with mononuclear cells, are found frequently. Laboratory measures of systemic inflammation usually return to normal by 6 to 8 weeks after illness onset.
Cardiac Manifestations
Coronary Artery Abnormalities
Kawasaki disease causes coronary artery abnormalities in 15% to 25% of patients who are not treated in the acute phase of the disease with high-dose IVIG. Lesions may be observed by echocardiography as early as 1 week from the onset of fever, with further progression of aneurysms ensuing in the next 3 to 4 weeks. Given the difficulty in reaching diagnostic confirmation of the illness using the classic criteria, identification of those at higher risk for coronary disease and for whom early treatment could reduce extent of involvement has led investigators to focus on predictive factors for coronary artery abnormalities. Many studies have explored predictors of coronary artery aneurysms. Late diagnosis and treatment with IVIG is the most important modifiable risk factor.43 Infants younger than age 6 months are at the highest risk for developing aneurysms, even when they are treated within the first 10 days of illness. The youngest infants frequently present with incomplete or atypical features, further increasing their risk of aneurysms by delaying diagnosis and IVIG treatment. Older children are also at higher risk for coronary aneurysms, in part because care providers do not consider Kawasaki disease as high in the differential diagnosis of the older child with fever.44 Many studies have highlighted the association of coronary aneurysms with persistent and recrudescent fever, as well as with IVIG resistance.45,46 Greater derangement of laboratory measures of the acute-phase response, reflecting more severe vasculitis, is also predictive and include lower hematocrit or hemoglobin (Hb), lower serum albumin, lower serum sodium, higher alanine aminotransferase, higher CRP and ESR, lower baseline serum IgG, and elevations in interleukin (IL)-6, IL-8, and other biomarkers.47–49 In addition, genetic polymorphisms (e.g., MMP haplotypes,29 polymorphisms of vascular endothelial growth factor [VEGF] and its receptors50) affect host susceptibility to aneurysms.
Myocarditis
In acute Kawasaki disease, myocarditis is a frequent finding at autopsy and by biopsy.51,52 Gallium-67 citrate scans53 and technetium-99 m-labeled WBC scans54 have also identified inflammatory myocardial changes in 50% to 70% of patients. Congestive heart failure (CHF) in the acute phase of the illness is generally the result of myocarditis and improves rapidly with IVIG treatment, in many cases within 24 hours from initiation of treatment.55 Later implications of these early changes is speculative, but several investigators have evaluated pathology and clinical function late after Kawasaki disease. Myocardial biopsies in patients with long-term follow-up have shown fibrosis, abnormal branching, and hypertrophy of myocytes unrelated to duration from illness onset.56 Although late noninvasive studies of myocardial function are encouraging, data on long-term myocardial function should accrue as the earliest Japanese cohorts reach middle age.57
Valve Regurgitation
Mitral and aortic regurgitation have been associated with Kawasaki disease in both early and chronic phases of the disease. In the acute stage, one in four children has mitral regurgitation of mild to moderate severity detected by two-dimensional (2D) echocardiography, which resolves in most children by the convalescent phase.58 The frequency of aortic regurgitation has been reported to be as high as 5% in Japanese children59 but only 1% in a recent North American population with Kawasaki disease,58 and there are rare reports of late-onset aortic regurgitation necessitating valve replacement.60,61 The cause for aortic insufficiency is not known; however, it has been reported that the aortic root enlarges from baseline and remains dilated during the first year after illness onset,58,62 raising the possibility that coaptation of the leaflets is disturbed.
Other Cardiac Findings
Rarely, patients in the acute or subacute phase of Kawasaki disease can develop tamponade due to a pericardial effusion, although effusions greater than 1 mm occur in fewer than 5% of patients.58,63 Rupture of a giant aneurysm into the pericardial space is an even rarer cause of pericardial tamponade.64–66
Cardiac Testing
Using 2D echocardiography, visualization of the proximal coronary arteries in young children is almost always possible, and measurements correlate closely with those identified by angiography. In addition to measurements of the proximal left main, anterior descending, circumflex, proximal, and middle right and posterior descending branches, assessment of ventricular function, pericardial fluid, and mitral and aortic regurgitation should be obtained. Coronary artery dilation may be present as early as the end of the first week of illness. In addition, findings on echocardiography are used to determine the need for IVIG treatment of children with suspected Kawasaki disease on the basis of fever and incomplete criteria.2 As a result, echocardiography should be undertaken when the diagnosis is entertained, particularly as an incomplete clinical picture, with coronary artery abnormalities sufficient to make the diagnosis and initiate therapy.2,67 Serial studies are obtained at 10 to 14 days from onset of illness to assess for the presence of aneurysm development and at the end of the subacute phase, 6 to 8 weeks from onset. Echocardiography should be performed more frequently in the child with coronary dilation at baseline, persistent fever, or other factors raising the likelihood of development of coronary aneurysms. Finally, children with giant aneurysms are at highest risk for development of coronary thrombosis in the first months after illness onset. For this reason, echocardiography may be performed once or twice a week until 8 weeks after illness onset or until coronary arteries stop enlarging and systemic inflammation has subsided.
The definition of normal coronary artery dimensions has been the subject of some controversy. Criteria established by the Japanese Ministry of Health in 1984 defined as abnormal those coronary arteries with lumen diameter greater than 3 mm in children younger than 5 years, or greater than 4 mm in those older than age 5; lumen diameter 1.5 times the size of an adjacent segment; or irregular lumen.68 Others have shown that coronary artery size in normal children correlates linearly with increasing body size. In patients with Kawasaki disease whose coronary arteries are classified as normal by Japanese Ministry of Health criteria, the dimensions are larger than expected when adjusted for body surface area (BSA) in all phases of the disease.69 Indeed, the median coronary z score at presentation in a multicenter trial was 1.43, significantly larger than the expected population norm in afebrile children of 0.49 Of note, normative data for febrile children are not available.
Echocardiography in the acute phase of Kawasaki disease is also useful for assessing left ventricular (LV) dysfunction.55 Among patients without coronary aneurysms, systolic function rapidly improves following IVIG administration.58 Diastolic function, specifically relaxation, is impaired in acute Kawasaki disease; patients with coronary aneurysms may continue to have long-term diastolic dysfunction, even when systolic function is preserved.70
Although echocardiography has high sensitivity and specificity for detection of dilation in the proximal coronary arteries, it is less useful for detection of coronary stenoses and aneurysms in the distal coronary vasculature. The quality of coronary imaging by echocardiography also diminishes as children grow larger, limiting its utility in long-term follow-up of the patient with Kawasaki disease. Therefore, imaging of the coronary arteries by ultrafast computed tomographic angiography (CTA) and magnetic resonance angiography (MRA) are used to obtain high-resolution images.71–75 Because the long-term effects of ionizing radiation are of special concern in children, CTA is used sparingly. Although the quality of coronary artery images may be superior with CTA, MRA does not require ionizing radiation and, in the child who is too young to exercise on a treadmill or bicycle, can be used with dobutamine or adenosine stress to delineate reversible ischemia.
Stress testing for inducible ischemia is an important component of periodic testing for patients with Kawasaki disease, but literature is limited to case series.76–79 As in so many other domains of care of the child with this disease, choice of stress test type is based upon literature related to adults with atherosclerotic coronary disease. As already noted, additional considerations include risks of repeated exposure to ionizing radiation and inability of the youngest children to exercise on a treadmill or bicycle, in whom pharmacological stress is required. To avoid false-positive test results, stress testing should only be performed in children with a history of coronary artery aneurysms.
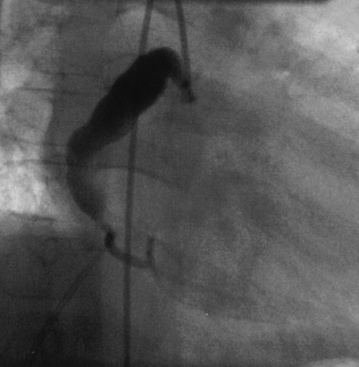
Coronary angiography has been used extensively for diagnostic assessment of coronary abnormalities. With rapid technical improvement in noninvasive imaging modalities and concerns about the use of ionizing radiation in children, invasive studies are generally reserved for children with aneurysms in whom noninvasive testing is insufficient to guide treatment or in whom revascularization is needed. Aneurysms are described as localized or extensive, the former being further subclassified as fusiform or saccular (Fig. 45-1). Extensive aneurysms, those that involve more than one segment, are ectatic (dilated uniformly; Fig. 45-2) or segmented (multiple dilated segments joined by normal or stenotic segments). Aneurysms may also involve other medium- to large-sized extraparenchymal arteries, particularly the subclavian, axillary, femoral, iliac, renal, and mesenteric arteries. Occasionally, aneurysms of the aorta may occur.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree