Fig. 11.1
Junctional ectopic tachycardia. The AH interval is 240 ms during the junctional ectopic tachycardia, which has a cycle length of 290 ms. The long AH interval and short or negative VA interval is consistent with both typical AV node reentry tachycardia (AVNRT), where antegrade conduction proceeds down the slow pathway and junctional tachycardia
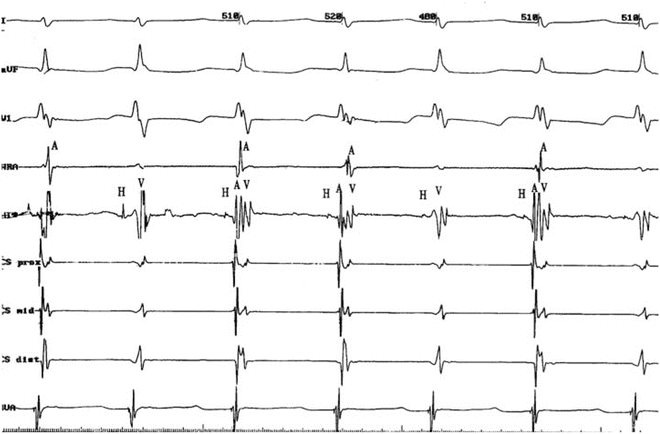
Fig. 11.2
JET with V-A dissociation. V-A dissociation without interruption of the tachycardia is most apparent in the HRA and CSp tracings. HRA high right atrium, His His bundle, CSp coronary sinus proximal, CSd coronary sinus distal, RVA right ventricular apex
Junctional tachycardia is not reproducibly initiated or terminated with programmed stimulation. Tachycardia initiation is not dependent on a critical A-H interval. During junctional tachycardia, a carefully timed single premature atrial complex (PAC) delivered during His refractoriness does not affect the timing of the subsequent His. In the case of AVNRT, a carefully timed single delivered during His refractoriness will conduct antegradely down the AV nodal slow pathway resulting in either perturbation of the subsequent His or termination of tachycardia (Chap. 3, Fig. 3.22). The ability to advance the immediate His with PAC placed before depolarization of the junctional area is 100 % specific for junctional tachycardia. If atrial overdrive pacing at a cycle length shorter than that of the tachycardia shortens the AV interval, the presence of dual AV node pathways (and thus AVNRT) can be eliminated (Fig. 11.3). Response to cessation of atrial pacing demonstrating an A-H-H-A response indicates junctional tachycardia, similar to V-A-A-V response of atrial tachycardia to ventricular overdrive pacing and A-H-A response during AVNRT.
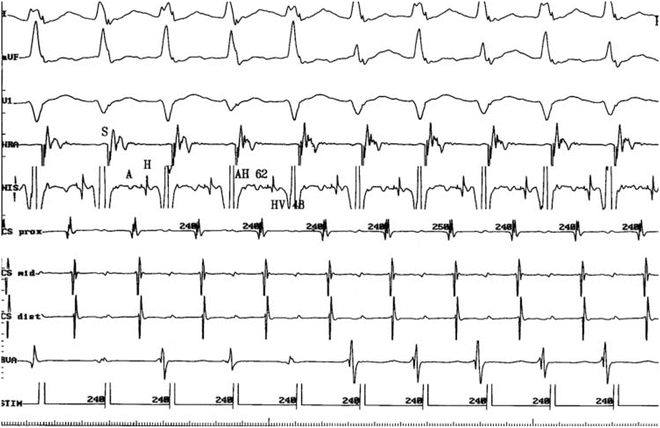

Fig. 11.3
Atrial pacing at a slightly faster rate (cycle length 240 ms) reduces the AH interval to 62 ms. This eliminates slow pathway conduction as a consideration, thereby excluding typical AVNRT as a possible mechanism
Postoperative Junctional Ectopic Tachycardia
Natural History
Postoperative JET (PO-JET) is the most common form of an abnormal automatic rhythm in children. The exact mechanism for this arrhythmia is unknown; however, the fluid and electrolyte shifts of the early postoperative state, trauma, stretch, local edema, or ischemia in the region of the AV node and/or His bundle are likely candidates. Similar to the non-postoperative forms of junctional tachycardia, PO-JET is an automatic arrhythmia displaying warm-up and cool-down transitions to sinus rhythm. The onset is typically during the first 72 h post-op and in the great majority of patients, during the first 24 h. The heart rate during PO-JET can range from 150 to 240 bpm, potentially compromising cardiac output (Fig. 11.4). The atria and ventricles are activated nearly simultaneously from impulses originating within the His bundle causing the contraction of the atria against closed atrioventricular valves (Fig. 11.5). This lack of atrioventricular synchrony compromises the cardiac output by decreasing ventricular filling. Uncontrolled, this tachycardia may further lead to a fall in blood pressure, initiating a downward spiral characterized by increased endogenous catecholamines and increased inotropic support to maintain adequate blood pressure and renal perfusion. As a result of vasoconstriction, the patient’s skin may feel cool, but the core temperature rises because of an inability to dissipate heat. This increase in endogenous and exogenous adrenergic input as well as the elevation in core temperature exacerbates the tachycardia. Thus, it is critical to interrupt this cycle of escalating heart rate, increasing vasoconstriction, and falling cardiac output. Although postoperative JET is often self-limited, slowing to a tolerable heart rate within 48 h, rapid rates (≥180–190 bpm) associated with decreasing blood pressure require intervention. The slower rates often do not need treatment or only need atrial pacing at a slightly higher rate than the junctional rate to provide atrial-ventricular synchrony, and they rarely persist beyond 5–7 days.
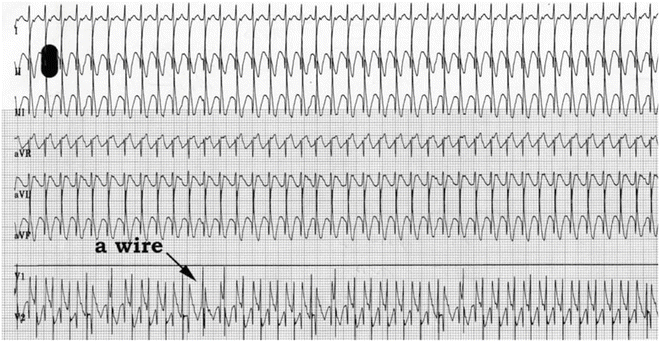
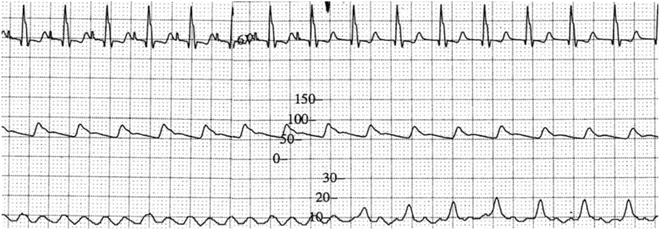

Fig. 11.4
Postoperative JET in 4-month-old girl following a repair of an atrioventricular septal defect (complete). The heart rate is approximately 260 bpm. Note the atrioventricular dissociation clearly demonstrated by recording of the atrial electrogram through the transthoracic temporary atrial epicardial electrode “a wire”; the “a” rate is approximately 160 bpm; there is variable retrograde conduction from (or capture of the atrium by) the ectopic junctional focus to the atrium. She responded to amiodarone and had an otherwise normal postoperative course. This illustration underscores the importance of postoperative transthoracic wires in diagnosing arrhythmias

Fig. 11.5
ECG tracing, blood pressure (middle), and right atrial recording (bottom) in a 2-year-old post-Fontan patient in the intensive care unit. In the left-hand part of the tracing, there is sinus tachycardia (CL = 410 ms) with a blood pressure of 85/50 mmHg, and a right atrial pressure of peak 10 mmHg. In the right-hand part of the tracings, there is junctional rhythm (CL = 430 ms) with “canon” waves of 15–20 mmHg and a blood pressure of 75/50 mmHg, due to loss of atrioventricular synchrony and thus atrial “kick”
The natural history of postoperative JET is time-dependent resolution, regardless of treatment, usually within 24–48 h. Early reports cited up to a 16% mortality. More recently with improved understanding and treatment, a fatal outcome is now uncommon, though there is an increased risk of perioperative death in patients experiencing JET compared to those without JET.
Incidence and Risk Factors
Many studies over the past two decades have attempted to establish the incidence and risks of developing postoperative JET. The reported incidence of JET varies widely from 1.4 to 15 % when all open cardiac operations for congenital malformations are considered. However, a number of lesions are the predominate ones at risk, including tetralogy of Fallot (14–37 %), repair of anomalous pulmonary venous return (36 %), arterial switch operation (23 %), atrioventricular canal (10–21 %), Norwood procedure (20 %), and interrupted aortic arch repair (17 %). Even Blalock–Taussig shunt placement has been reported to result in PO-JET, despite the fact that no intracardiac manipulation is performed. Other risk factors are longer duration of cardiopulmonary bypass, longer aortic cross clamp time, patient temperature, and elevated biomarkers of cardiac injury. Inotrope use, especially dopamine and milrinone, have been correlated to higher risk of JET. Specific genetic polymorphisms, such as the angiotensin-converting enzyme insertion/deletion polymorphism also appear to predispose to JET.
The wide variation in incidence is likely dependent on multiple factors, including an era effect and different center practices. One major cause of variation is the lack of a standard definition of JET across these studies. Early studies on PO-JET differentiated it from other tachyarrhythmias by failure of overdrive pacing or DC cardioversion. Even today the precise definition of JET remains unclear—either exclusively having VA dissociation or inclusive of both VA dissociated and 1:1 conducting arrhythmias provided the tachycardia is diagnosed “JET” in the patient’s medical record. The former criteria will underdiagnose those patients who conduct 1:1 retrograde consistently during JET. The latter interpretation will overdiagnose JET, including other normal complex QRS tachycardias with 1:1 conduction and simultaneous VA or short VA conduction. Visualization of the onset or offset of the tachycardia or other diagnostic maneuvers (overdrive pacing, adenosine) is necessary for a completely accurate diagnosis.
Treatment
The treatment of postoperative JET has evolved over the past two decades. The initial steps are reduction of catecholaminergic stimuli along with, hypothermia, atrial pacing, antiarrhythmic medications, and time.
Hypothermia induction to 32–34° using extracorporeal cooling or intravenous cooling is effective for slowing the rate. This strategy is also used to allow atrial pacing at 10–20 bpm above the JET rate, thereby restoring atrioventricular synchrony. The JET rate typically needs to be <180 bpm to successfully overdrive the rhythm. This goal can also be achieved with antiarrhythmic medications.
Nearly all intravenous antiarrhythmic medications have been evaluated as therapy for PO-JET. The earliest studies showed that digoxin has some effect, but beta-blockers, phenytoin, and calcium channel blockers were found to be relatively ineffective and carried an increased risk of complication due to their negative inotropic effects. The class IC antiarrhythmics, flecainide, and propafenone have been the subjects of favorable reports in small single center studies. Importantly, procainamide reduces JET rates in a dose-dependent manner. Despite the demonstration of efficacy and the absence of side effects in these prospective studies, procainamide may lead to significant causing hypotension secondary to sympathetic ganglion blockade.
Amiodarone is the most widely studied antiarrhythmic for the treatment of PO-JET. It appears most efficacious when administered as 1 or 2 loading doses of 5 mg/kg followed by a continuous infusion. A double-blind randomized controlled drug trial evaluated the efficacy of three different amiodarone dosing strategies on incessant tachyarrhythmias in pediatrics. While a dose-dependent time to effect was found for the entire dosing level cohorts, JET as a subgroup did not show significant difference in efficacy across dosing levels cohort. The study also found a dose-dependent risk of side effects: 36 % of patients experienced hypotension (undefined); 20 %, bradycardia (undefined); and 15 %, atrioventricular conduction block. Amiodarone has also been used prophylactically after tetralogy of Fallot repair as a 48 h continuous infusion (2 mg/kg/day) with a decrease in the occurrence of JET (10 %) as compared to a control group (37 %). It is important to remember the high incidence of acute, chronic, and potentially severe side effects of amiodarone when considering it as a treatment strategy for this self-limited arrhythmia.
Magnesium supplementation during cardiopulmonary bypass for repair of congenital heart defects (unspecified) was the subject of a prospective randomized double-blind trial to reduce PO-JET. It reduced the incidence of PO-JET in the high dose group, 50 mg/kg of magnesium sulfate (n = 40) to zero. Dexmedetomidine, an alpha-2-adrenergic receptor agonist has shown some early promise as a treatment as well.
Many centers employ a staged treatment protocol to address PO-JET, utilizing a sequential combination of treatments. Initial interventions include reduction of inotropic support and catecholaminergic stimuli, if possible, including sedation and paralysis. Cooling followed by active cooling and antiarrhythmic medications are instituted subsequently. Atrial pacing above the JET rate should be considered at any stage. The rapidity that one moves through the levels of treatment is dependent on the patient’s clinical status and response to intervention. Because PO-JET is a transient arrhythmia, it is important to weigh the use of treatment with the clinical need. Stable PO-JET, without significant hemodynamic consequence, can be treated solely by removal of stimuli, cooling, and patience.
Congenital Junctional Ectopic Tachycardia
Congenital JET (Fig. 11.6) is a rare arrhythmia occurring in patients 6 months of age or younger, not associated with a cardiac operation. Affected infants often present with symptoms of congestive heart failure or fetal hydrops in the setting of an incessant tachycardia. Some infants may initially appear compensated only to subsequently develop cardiovascular collapse. Patients with congenital JET are most likely to have incessant tachycardia and more likely to have sustained rather than sporadic tachycardia. The heart rate typically is >200 bpm, although it may be as slow as 130–150 bpm. Criteria for diagnosis are identical to those for PO-JET, but given the rarity of the disorder, a high index of suspicion is needed.
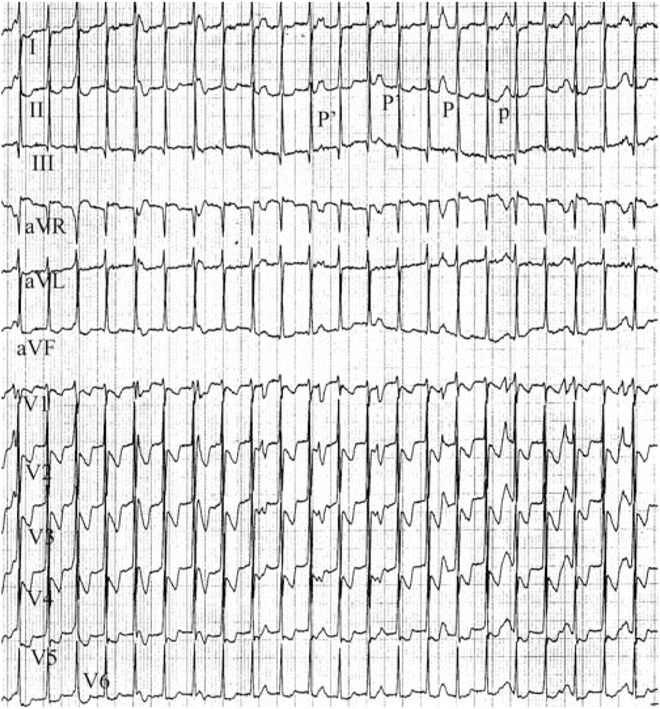

Fig. 11.6
12-Lead ECG of congenital JET in a 9-month-old girl. Note the narrow QRS tachycardia at 170 bpm and AV dissociation (large P-waves in lead II. Note brief period of 2:1 retrograde conduction to the atria (P′). This girl was treated with amiodarone for 12 months and the arrhythmia subsided. The amiodarone was discontinued and JET has not returned after 9 years
Congenital JET is associated with a relatively high mortality risk. The incessant nature of the tachycardia may lead to secondary cardiomyopathy and congestive heart failure. An early large multicenter report showed an overall mortality rate of 35 % in 27 congenital JET patients gathered over 17 years. In a larger, more recent cohort, mortality was 9 %. Those patients who do not succumb to early hemodynamic compromise will show spontaneous slowing of the junctional rate; yet follow-up Holter evaluation >5 years after apparent symptom resolution may show persistent JET at slower rates during periods of sleep when the sinus rate slows.
Incidence and Risk Factors
Congenital JET is rare. A review of all non-postoperative JET patients from 22 centers over 40 years identified just 44 patients with tachycardia at 6 months or less. Maternal and neonatal risk factors are unclear due to the rarity of the diagnosis. Fetal tachycardia was found in 36 % of the congenital JET population and an association with family history of JET and minor congenital heart defects was noted. The influence of maternal auto-antibodies is unknown.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


