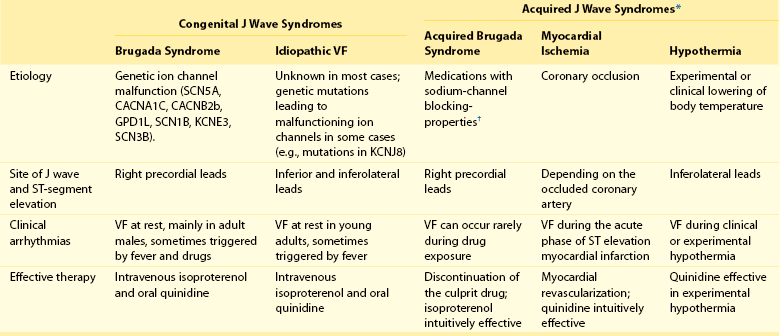
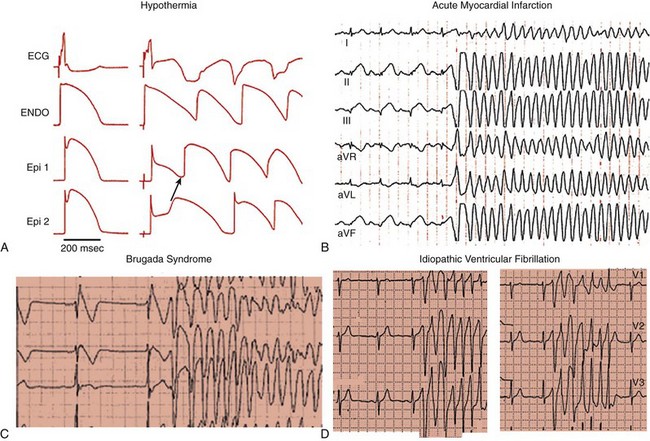
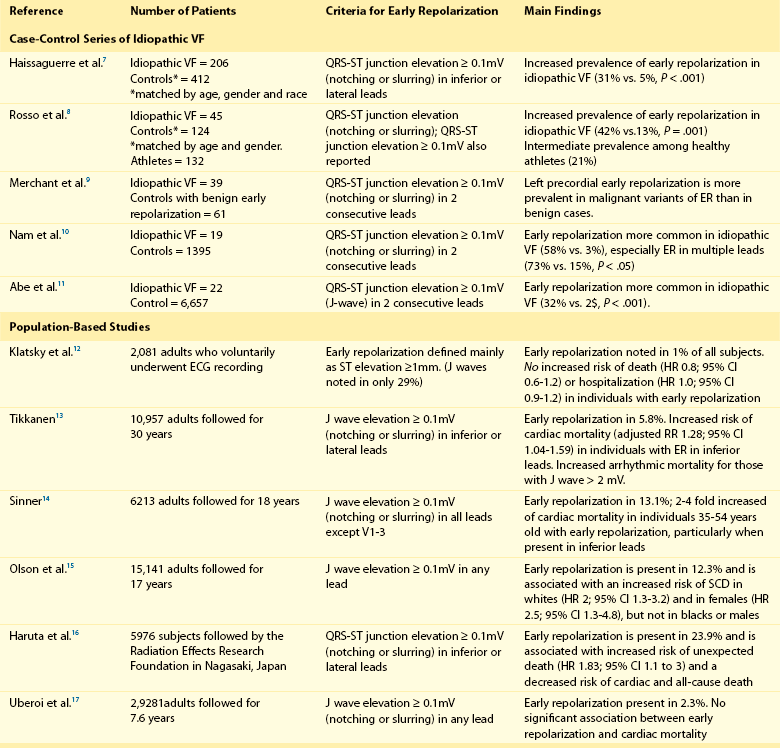
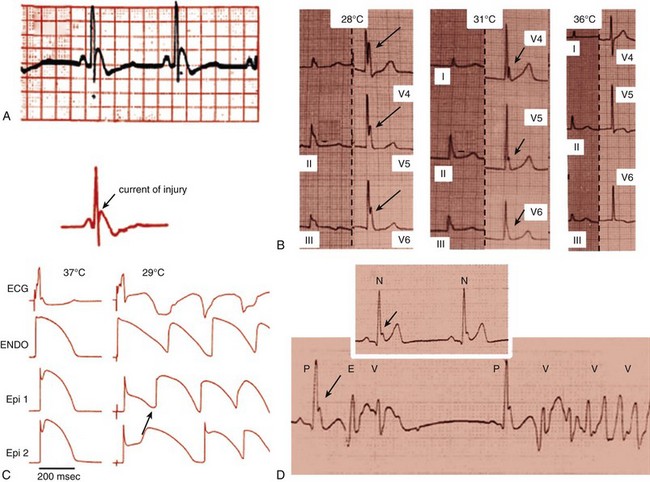
96 Acquired J Wave Syndromes: Hypothermia and Ischemic Ventricular Fibrillation Congenital J Wave Syndromes: Brugada Syndrome and Idiopathic Ventricular Fibrillation Early Repolarization in Healthy Individuals and J Waves in Idiopathic Ventricular Fibrillation Arrhythmic Risk for Patients with Early Repolarization in Large Prospective Population Studies The term J wave syndromes denotes a clinical spectrum consisting of diseases that greatly differ from each other in terms of etiology and clinical characteristics, but share similar electrocardiographic (ECG) features and arrhythmogenic mechanisms.1 Etiologies include genetic disorders (Brugada syndrome), acquired disorders (myocardial ischemia and hypothermia), and diseases of unclear etiology (idiopathic ventricular fibrillation [VF] with early repolarization; Table 96-1). The arrhythmogenic mechanism shared by these diseases is an uneven abbreviation of the action potential (in some myocardial areas more than others). The resulting increased dispersion of ventricular repolarization underlies the ECG hallmark of these disorders: J-point elevation (also termed J wave) and ST segment elevation. When the dispersion of ventricular repolarization reaches a certain threshold (i.e., when areas of markedly different action potential duration coexist in contiguous myocardial areas), malignant reentrant polymorphic ventricular tachyarrhythmias occur (Figure 96-1, A). This common arrhythmogenic mechanism explains why disorders with fundamentally different etiologies, such as an inborn malfunction of cardiac sodium channels in Brugada syndrome versus acute coronary occlusion in the case of acute myocardial infarction, cause ventricular arrhythmias of identical morphology. As shown in Figure 96-1, arrhythmias in the various J wave syndromes are polymorphic and invariably start with a short-coupled ventricular extrasystole. Table 96-1 *The distinction between congenital and acquired forms is not always straightforward (see text). Figure 96-1 Arrhythmias in the J wave syndromes. A, Experimental hypothermia in the wedge preparation model.2 At baseline, the action potential is slightly shorter in the epicardium than in the epicardium, because the former normally has larger ITo currents; however, the dispersion of repolarization (i.e., the difference between the shortest and longest action potential duration) is small. Cooling of the preparation leads to shortening of the action potential; however, this action-potential shortening is disproportionally accentuated in epicardial layers, leading to loss of the action potential dome. Note that the action potential dome is still present in the endocardium at a time when the epicardium is no longer refractory. This allows for propagation of the action potential dome from endocardium to epicardium (phase 2 reentry). B, C, and D, Spontaneous initiations of ventricular fibrillation (VF) in a patient with acute inferior myocardial infarction (note the ST-segment elevation in leads II, III, and aVF in B), in a patient with Brugada syndrome (C) and a patient with idiopathic VF (D). Note that in all cases VF is initiated by a ventricular extrasystole with short coupling interval (on the T wave of the last sinus complex). (A, Reproduced from Fish JM, Antzelevitch C: Link between hypothermia and the Brugada syndrome. J Cardiovasc Electrophysiol 15:942, 2004.) Three points are noteworthy. First, although some J wave syndromes (e.g., idiopathic VF or hypothermia) are entirely due to repolarization abnormalities, depolarization delay is important in others. Specifically, conduction delay to the right ventricular outflow tract (RVOT) and through ischemic myocardial zones, contribute to the arrhythmias of Brugada syndrome,3 and ischemic VF.4 Second, although J wave syndromes are classified as congenital or acquired (see Table 96-1), both characteristics can coexist. For example, patients with congenital mutations not severe enough to manifest Brugada syndrome are predisposed to develop VF when challenged with specific drugs (http://www.brugadadrugs.org/) or acute myocardial infarction.5 In addition, patients with idiopathic VF can develop recurrent VF during therapeutic hypothermia.6 Third, it is important to emphasize that J waves are often observed in healthy individuals who have the early repolarization ECG pattern. Although this ECG pattern has been considered benign, case-controlled series recently demonstrated a significant association between the early repolarization pattern and idiopathic VF (Table 96-2). It is therefore imperative to distinguish the common and benign early repolarization pattern from the rare and malignant ECG of the J wave syndromes. Table 96-2 Evidence Supporting the Association Between Early Repolarization and Increased Risk for Arrhythmic Death Modified from Rosso R, Adler A, Halkin A, Viskin S: Risk of sudden death among young individuals with J waves and early repolarization: putting the evidence into perspective. Heart Rhythm 8:923–929, 2011. In this regard, it is important to note that the early repolarization pattern has conventionally been defined as the combination of J waves with ST segment elevation. However, recent evidence suggests that each of these components has different prognostic connotation.18,19 As explained in this chapter, J waves without ST segment elevation can be particularly arrhythmogenic.20 As a result, this chapter summarizes the clinical and ECG features of the J wave syndromes with an emphasis on the morphology of the J waves and the contour of the ST segments. A detailed explanation of the arrhythmic mechanisms of J wave syndromes appears in Chapter 52. The prototype of the arrhythmogenic J wave was described by Osborn; dogs subjected to worsening degrees of hypothermia developed increasingly taller J waves and eventually VF.21 This ECG hallmark of impending VF involves giant J waves without ST segment elevation (Figure 96-2, A), and the same applies to clinically significant hypothermia in humans22 (see Figure 96-2, B). Experiments involving cooling of cardiac-wedge preparations demonstrated that hypothermia-related J waves are the ECG reflection of increased dispersion of repolarization caused by disproportionate abbreviation of the epicardial action potential, eventually leading to phase 2 reentry and VF (see Figure 96-2, C).2 Quinidine is extremely effective for preventing hypothermia-induced VF in animal studies,24 which is an interesting finding considering that quinidine is the most effective drug for preventing VF in clinical J wave syndromes.25,26 Figure 96-2 The arrhythmogenic J wave. A, The J waves recorded by Osborn during his experiments with hypothermia in dogs, including his original annotation: “current of injury.”21 Dogs with this injury current went on to develop spontaneous ventricular fibrillation (VF). Note that the marked J waves of hypothermia are not accompanied by segment elevation (ST-E), neither in experimental hypothermia (A) nor in clinically significant hypothermia in humans (B).22 C, The wedge-preparation experiment demonstrating that hypothermia-induced J waves are the electrocardiographic representation of increased dispersion of repolarization. Decreasing the temperature of the coronary perfusion solution from 37° C to 29° C augments the action potential notch in epicardium, but not endocardium, and increases the amplitude of the J wave. The cooler temperature leads to heterogeneous loss of the epicardial action potential dome, phase 2 reentry within the epicardium (arrow), and polymorphic ventricular tachycardia.1 D, The electrocardiogram of a patient with idiopathic VF.23 Note that the J wave amplitude increases after pauses and immediately before the onset of VF; however, J wave augmentation is not accompanied by ST elevation. (Entire figure reproduced from Viskin S, Rosso R, Halkin A: Making sense of early repolarization. Heart Rhythm 9:566–568, 2012; A, reproduced from Osborn JJ: Experimental hypothermia; respiratory and blood pH changes in relation to cardiac function. Am J Physiol 175:389–398, 1953; B, reproduced from Kanna B, Wani S: Giant J wave on 12-lead electrocardiogram in hypothermia. Ann Noninvasive Electrocardiol 8:262–265, 2003; C, reproduced from Fish JM, Antzelevitch C: Link between hypothermia and the Brugada syndrome. J Cardiovasc Electrophysiol 15:942–944, 2004; D, reproduced from Shinohara T, Takahashi N, Saikawa T, Yoshimatsu H: Characterization of J wave in a patient with idiopathic ventricular fibrillation. Heart Rhythm 3:1082–1084, 2006.) The mechanisms underlying ischemic arrhythmias are more complex4; however, VF occurring during acute ST elevation myocardial infarction also appears to be due to phase 2 reentry.4 In the setting of acute coronary occlusion, the fall in the action potential dome in the ischemic zone occurs principally because of activation of adenosine triphosphate (ATP)-sensitive potassium current (IK-ATP) secondary to a reduction in intracellular ATP levels and increase in adenosine diphosphate levels because of tissue ischemia. Phase 2 reentry occurs secondary to propagation of the action potential dome from normal zone to ischemic regions.4
J Wave Syndromes
Acquired J Wave Syndromes: Hypothermia and Ischemic Ventricular Fibrillation
Thoracic Key
Fastest Thoracic Insight Engine



