CHAPTER 91 Ischemic Mitral Regurgitation
Definition
Carpentier’s pathophysiologic triad1 is our preferred approach for defining ischemic mitral regurgitation. Most of the clinical literature on ischemic mitral regurgitation remains difficult to interpret because imprecise definitions are often applied, which leads to marked heterogeneity within and between patient populations.2 We feel strongly that a precise definition of ischemic mitral regurgitation according to the pathophysiologic triad is critical to the presentation of a relatively homogeneous group of patients for analysis and treatment. A strict definition of ischemic regurgitation according to the triad typically requires the following:
The majority of patients with ischemic mitral regurgitation have so-called functional ischemic mitral regurgitation from IIIb dysfunction without any leaflet or subvalvular lesions (leaflet tethering occurring because of papillary muscle displacement). Although the term functional mitral regurgitation is commonly used to refer to mitral regurgitation without “organic” lesions of the mitral valve, its use should be discouraged, because it is an imprecise term and lacks the mechanistic and therapeutic correlates of the Carpentier functional classification. Furthermore, although it is often assumed that the valve apparatus is normal in functional regurgitation, pathologic studies have shown that these leaflets are thinned out with altered collagen composition compared with autopsy controls.3
The mitral regurgitation grade should be at least mild by semiquantitative or quantitative assessment, preferably by transthoracic echocardiography performed while the patient is awake and not actively ischemic. General anesthesia and even the light sedation required for transesophageal echocardiography (TEE) can unload the ventricle and lead to underestimation of the degree of mitral regurgitation.4 The potential unloading effects of inotropes, vasodilators, and intra-aortic balloon pump should also be taken into consideration. During the past 2 decades, state-of-the-art techniques for quantifying mitral regurgitation by echocardiography have emerged, and they are particularly useful for patients with ischemic mitral regurgitation. The recommended method is to calculate the regurgitant volume and the effective regurgitant orifice using the flow-convergence proximal isovelocity surface area (PISA) method.5 The Mayo group has suggested that the thresholds for defining significant mitral regurgitation should be lower (regurgitant volume, ≥30 mL; effective regurgitant orifice area, ≥20 mm2) in functional mitral regurgitation than in organic mitral regurgitation, given its impact on survival in this group.6 Cine ventriculography has been superseded by echocardiography for diagnosis and grading of ischemic mitral regurgitation. Absence of mitral regurgitation on ventriculography should not be regarded as sufficient to exclude ischemic mitral regurgitation—this is increasingly important in the current era, as many interventionalists have moved toward low-volume injection technique for ventriculography, which is suboptimal for assessing mitral regurgitation.7
Clinical Presentation
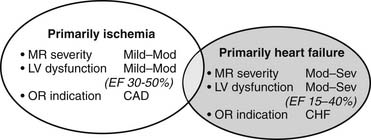
Patients with type IIIb ischemic mitral regurgitation, therefore, typically present with one of two clinical scenarios (Fig. 91-1). The more common scenario is a patient with symptomatic multivessel coronary artery disease, with or without associated congestive heart failure symptoms, who is referred for surgical or percutaneous revascularization and who is noted to have mild to moderate mitral regurgitation on preoperative or intraoperative imaging. The other scenario is a patient with moderate to severe mitral regurgitation and primarily congestive heart failure symptoms, who is referred for mitral valve surgery and who also has significant coronary artery disease. Increasingly, patients in the latter group have undergone prior percutaneous or surgical revascularization. Associated coronary artery disease typically involves more than one vessel, but ischemic mitral regurgitation can also occur in the setting of single-vessel disease of any of the three main coronary arteries.
PATHOPHYSIOLOGY
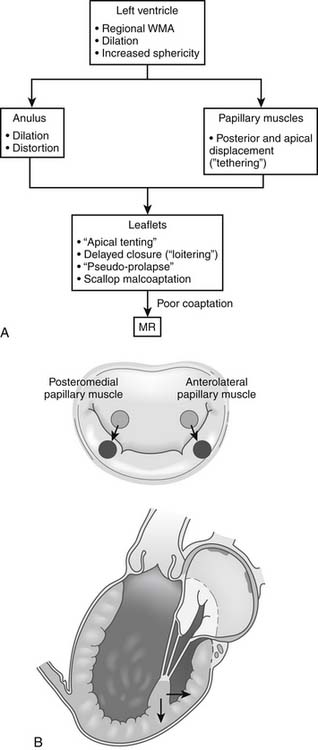
The key pathophysiologic components of types I and IIIb ischemic mitral regurgitation include anular changes (dilation, distortion), subvalvular changes (papillary muscle displacement), and ventricular changes (dilation with increased sphericity, and wall motion abnormalities). Figure 91-2 provides an overview of how these components interact to result in poor leaflet coaptation, which is the final common pathway for all types of mitral regurgitation.
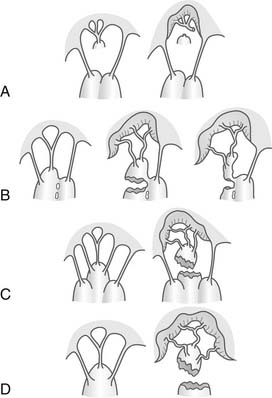
Figure 91–14 Classification of papillary muscle rupture according to Jouan, et al.108 A to C represent partial rupture and typically present in subacute or chronic fashion. D is the more classic papillary muscle rupture. Mechanisms of ischemic mitral valve prolapse shown. A, Necrosis of a separate commissural head (inserted close to the anulus) with rupture of the anchorage of the commissural chord. B, Necrosis of a single head papillary muscle subdivided in multiple heads with partial rupture. C, Necrosis of a fenestrated papillary muscle with detachment of its main insertion: “incomplete” rupture. With time, incomplete rupture mimics papillary muscle elongation. D, Single papillary muscle with complete and total rupture.
Specific Geometric Abnormalities
Anular
Although anular dilation is a common finding in clinical cases of chronic ischemic mitral regurgitation, the degree of dilation varies and does not necessarily correlate with the degree of mitral regurgitation. Some patients with significant ischemic mitral regurgitation have very little anular dilation, and others with significant anular dilation may not have much mitral regurgitation. Laboratory data support the notion that anular dilation per se is not a fundamental component of ischemic mitral regurgitation pathophysiology. Green and colleagues8 showed that in an acute sheep model, moderate degrees of anular dilation do not necessarily lead to ischemic mitral regurgitation. Timek and coworkers9 noted that the mild degrees of anular dilation that they observed during acute occlusion of the left anterior descending or distal left circumflex did not result in ischemic mitral regurgitation. This study, and several others from the same group, noted the critical role of the septolateral (SL) dimension of the anulus in the pathophysiology of ischemic mitral regurgitation. The SL dimension—also referred to as the anteroposterior (AP) dimension—is the vertical or short axis of the anular ellipse that extends from the middle of the anterior anulus to the middle of the posterior anulus. The horizontal or long axis of the ellipse is referred to as the commissure-commissure (CC) dimension. These studies have shown that in the sheep acute ischemia model, an increase in mitral anular area causes mitral regurgitation only to the extent that it increases the SL dimension. In fact, the CC dimension does not usually increase significantly with ischemia.9–12 Furthermore, these researchers have shown that decreasing the SL dimension using a cinching device can abolish ischemic mitral regurgitation in sheep independent of overall anular circumference.11,12 In severe cases of ischemic mitral regurgitation, the SL dimension can approach the CC dimension, which results in a circular anulus instead of the normal elliptical one. Anular dilation is predominant in the posterior mitral anulus—hence the usual disproportionate increase in the SL compared with CC dimension. Although most of the dilation is in the posterior anulus, the fibrous anterior anulus can also dilate.13,14 This has been well demonstrated in three-dimensional echocardiographic and magnetic resonance imaging studies,10 which show that although anular dilation is predominant in the region of the posterior commissure, it is often asymmetrically present throughout the anulus.
Subvalvular
Papillary muscle displacement also plays a critical role in the pathophysiology of ischemic mitral regurgitation. It is important to distinguish this from papillary muscle dysfunction (decreased or discoordinate papillary muscle systolic shortening), which had previously been thought to be the primary mechanism for ischemic mitral regurgitation. In fact, papillary muscle dysfunction does not necessarily result in ischemic mitral regurgitation. Timek and coworkers15 showed that the decreased shortening of one or both papillary muscles did not correlate with ischemic mitral regurgitation in the sheep. On the contrary, Messas and colleagues16 demonstrated a paradoxical decrease in ischemic mitral regurgitation with papillary muscle dysfunction.
The primary alteration in papillary muscle geometry leading to ischemic mitral regurgitation is papillary muscle displacement. The pattern of displacement is actually fairly complex and cannot simply be described as apical tethering. The papillary muscle tips are displaced away from the mid-septal (anterior) anulus (i.e., posterolaterally, apically, and away from each other) (see Fig. 91-2B). The tethering distance has been shown experimentally to correlate with the severity of ischemic mitral regurgitation.9,15,17 Experimental studies suggest that although displacement of both papillary muscles is probably necessary to induce ischemic mitral regurgitation, displacement of the posteromedial papillary muscle usually predominates.
Left Ventricular
The initiating insult in ischemic mitral regurgitation is ventricular, specifically myocardial ischemia or infarction with remodeling that leads to regional anular and subvalvar distortion and ultimately poor leaflet coaptation. The independent contribution of global ventricular size and shape to the pathophysiology of ischemic mitral regurgitation is less clear. In an echocardiographic study of 102 patients, Kumanohoso and coworkers18 showed that in comparison to those with anterior infarctions, patients with inferior infarctions had a higher incidence of ischemic mitral regurgitation despite less severe left ventricular dilation and dysfunction; this was directly attributed to more severe posteromedial papillary muscle tethering in the inferior infarction group. Experimental data, however, suggest that regional left ventricular dysfunction does not lead to ischemic mitral regurgitation without some degree of left ventricular dilation.
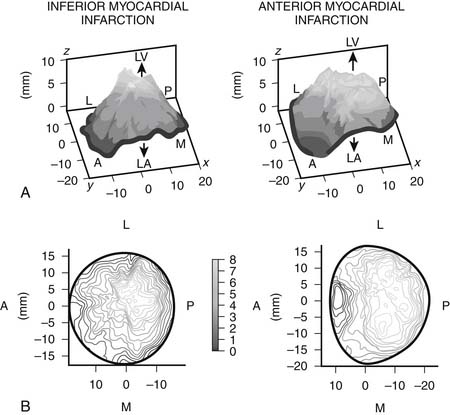
It appears that the left ventricular sphericity is more important than the actual ventricular volumes or ejection fraction in the pathophysiology of ischemic mitral regurgitation. Using three-dimensional echocardiography, several groups have shown that the mechanism of regurgitation differs according to the region of infarction. Watanabe and colleagues, for example, found that tethering was more localized and predominant in the medial posterior leaflet in patients with inferior infarction, compared with more widespread tethering of both leaflets in anterior infarction (Fig. 91-3).19 This observation confirms the critical role of regional ventricular geometry and function in the pathophysiology of ischemic myocardial regurgitation and helps explain why some patients with only mildly impaired global ventricular function have severe ischemic mitral regurgitation. Song and colleagues20 found that an inferior infarction coexisting with an anterior wall infarct led to a more severe regurgitation with an effect independent of ventricular volume. These observations suggest that geometry of the valve apparatus may be a more important determinant of regurgitation than left ventricular volume or ejection fraction. It is possible, therefore, to have severe ischemic mitral regurgitation in the setting of relatively preserved ventricular function. In those patients in whom inferior and anterior infarction coexist, tethering is more symmetrical.20
Leaflet Dysfunction
Papillary muscle tethering leads to apical tenting of the leaflets (restriction of the motion of the free margins of the leaflets), which prevents the free margins of the leaflets from rising to the plane of the anulus to coapt with one another. Tethering of the secondary chordae can result in an effet de mouette deformation of the body of the leaflet, which further impairs coaptation. Mal-coaptation of the scallops of the posterior leaflet from papillary muscle tethering has also been implicated in the pathogenesis of ischemic mitral regurgitation.21
Therapeutic Targets in Ischemic Mitral Regurgitation
Mitral Anulus
The mitral anulus has been the principal target of surgical therapy for ischemic mitral regurgitation. The restrictive (or downsized or undersized) anuloplasty is the currently recommended approach—anuloplasty corrects circumferential anular dilation, whereas downsizing corrects the septolateral displacement and thus reduces the tethering distance. Asymmetrical rings may be helpful in addressing the asymmetrical tenting seen in inferior infarction.22 Complete rigid or semirigid rings are preferred, as they ensure that the valve remains fixed in the new reduced SL dimension. Incomplete anuloplasty with flexible bands may not fix the SL dimension.
The differences in tethering between anterior and inferior infarctions lead to the question of whether anuloplasty rings should differ depending on location of infarction. Lack of emphasis of the anuloplasty on areas of greatest tethering may be responsible for some recurrences after surgical repair. For example, the Carpentier-McCarthy-Adams IMR ETlogix ring (Edwards Lifesciences, Irvine, CA) is designed to address asymmetrical tethering seen in inferior infarction,22 and it may not be as effective in anterior infarction, where the tethering is more widespread. Better understanding of three-dimensional anular geometry may lead to the development of anuloplasty techniques that are tailored to the individual patient based on specific anular geometry and tenting distribution.
Subvalvar Apparatus
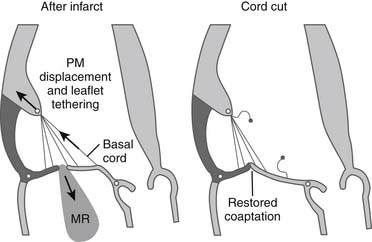
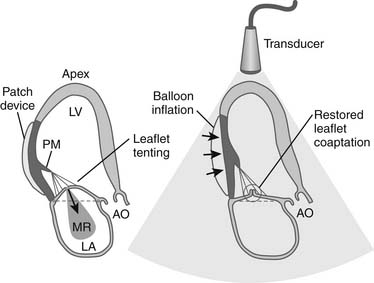
Subvalvar approaches aim to reduce leaflet tethering and thus allow better leaflet coaptation. Two broad approaches exist. Tethered secondary cords may be divided to improve leaflet mobility (Fig. 91-4). Very restrictive primary cords can also be divided and replaced with artificial chordae. Alternatively, the tethering distance between the papillary muscle heads and the anular plane may be reduced by using external or internal devices. Techniques have been developed that relocate the papillary muscles so that they are closer to the anulus (i.e., less displaced), thereby reducing the leaflet tethering. External prosthetic devices, studied in animals, work by displacing the infarcted ventricular wall, which supports the tethered papillary muscle, toward the mitral anulus, thus reducing tethering and resultant regurgitation (Fig. 91-5).23,24
Leaflets
Because the leaflets are not directly involved in the causation of ischemic mitral regurgitation, there is minimal scope for surgical intervention on the leaflets. Clefts tend to open up with leaflet tethering, and they should be closed at the time of mitral valve repair. The edge-to-edge leaflet technique has been proposed as an adjunct to anuloplasty,25 but alone it is not an effective treatment for ischemic mitral regurgitation.26
Ventricle
Operations to restore left ventricular geometry can decrease papillary muscle displacement and result in reduction of mitral regurgitation. This could be achieved by ventricular remodeling procedures (such as the Dor operation) or by localized plication of a lateral wall infarct. Approaches that limit continued remodeling may improve durability of ischemic mitral valve repair by limiting further papillary muscle displacement. For example, external ventricular restraint devices wrapped round the ventricle at the time of mitral valve repair may reduce future dilation of the left ventricle and thus eliminate or reduce one of the key pathophysiologic mechanisms for recurrent regurgitation after mitral anuloplasty.27 Injection of polymers into infarcted tissue has been explored experimentally by Hung and colleagues,28 who demonstrated that polymer injection supported the infarcted ventricle and stabilized the papillary muscles, thereby resulting in acute reverse remodeling of the ventricle and reduction in papillary muscle displacement and decrease in mitral regurgitation. Finally, injection of myoblasts or stem cells into infarcted tissue is another potentially beneficial approach that could reduce mitral regurgitation by restoring ventricular function and therefore reducing tethering.29
SURGICAL DECISION MAKING
Decision Making in Specific Clinical Scenarios
Moderate to Severe Ischemic Mitral Regurgitation with Congestive Heart Failure
The patient who presents with moderate to severe mitral regurgitation, symptoms of congestive heart failure, or worsening left ventricular function is referred primarily for mitral valve surgery. The preoperative coronary angiogram typically shows significant coronary artery disease that may or may not have been symptomatic (with angina). Patients may have undergone prior surgical or percutaneous revascularization. These patients typically have evidence of prior myocardial infarction and moderate or severe left ventricular dysfunction. They can be viewed as having mitral regurgitation as a result of ischemic cardiomyopathy. If the ejection fraction is particularly low (<30%), the decision to operate in the absence of documented ischemia is similar to the decision that needs to be made for patients with mitral regurgitation as a result of nonischemic cardiomyopathy. The surgical decision in this clinical scenario is more straightforward. Patients with moderate to severe ischemic mitral regurgitation with symptoms of congestive heart failure or worsening left ventricular function (or both) should undergo combined mitral valve surgery and CABG as long as the expected operative morbidity and mortality are not prohibitive. For patients who survive and recover from surgery, the expected benefit of surgery would be better control of heart failure with likely improvement of symptoms. However, there is no clear evidence that life expectancy is improved by mitral valve repair in the setting of left ventricular dysfunction, and indeed some data suggest the contrary.30 For this reason, younger patients with ischemic mitral regurgitation and severe left ventricular dysfunction, with New York Heart Association (NYHA) class III or IV heart failure, could alternatively be considered for heart transplantation.
Mild to Moderate Ischemic Mitral Regurgitation at Coronary Artery Bypass Grafting
The summation of best available evidence is in favor of intervening on moderate degrees of regurgitation at the time of CABG.46 We recommend anuloplasty for all patients having coronary artery bypass surgery who have documented moderate or greater mitral regurgitation unless specific operative or patient-related issues suggest a need for a more limited or expeditious operation. We would also recommend anuloplasty for patients with mild regurgitation, if the degree of regurgitation increases on intraoperative provocative testing or if the depth of coaptation is minimal.
Impact of Ischemic Mitral Regurgitation on Survival
After Myocardial Infarction
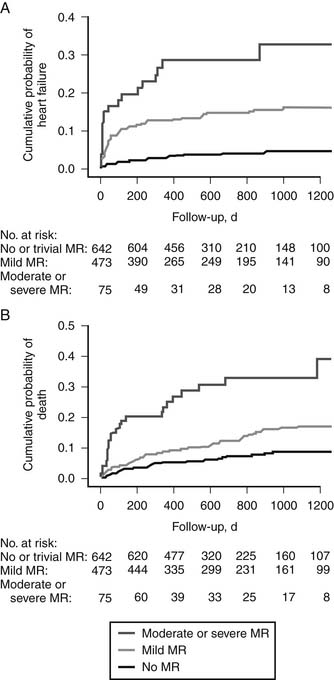
Several studies have documented the strong negative impact of mitral regurgitation on survival and the occurrence of heart failure after acute myocardial infarction. In their analysis of a subgroup of 727 patients with acute myocardial infarction from the Survival and Ventricular Enlargement (SAVE) trial, Lamas and colleagues47 found that even mild mitral regurgitation at the time of presentation was an independent predictor of mortality (relative risk, 2.0), and that this effect could not be attributed to differences in left ventricular function. Grigioni and coworkers6 found a direct correlation between survival and the severity of mitral regurgitation using quantitative echocardiography done within 6 weeks of myocardial infarction. More recently, Aronson and coworkers,48 reporting on 1190 patients who had sustained acute myocardial infarction, observed that that within 3 years, 30% of those with moderate or severe regurgitation had been hospitalized for heart failure, compared with 5% for those without regurgitation (Fig. 91-6A). The 3-year mortality was higher in those with moderate or severe regurgitation (35%) than in those without regurgitation (8%) (hazard ratio, 5.5). Even mild regurgitation was a risk factor for medium-term mortality (hazard ratio, 2.0) (see Fig. 91-6B). This suggests that little has changed from the earlier epidemiologic studies of the 1990s6 and that ischemic mitral regurgitation after myocardial infarction remains associated with substantial burden of mortality and heart failure. This negative effect is independent of left ventricular function, as patients with moderate or greater regurgitation and preserved ventricular function also incur excess mortality and morbidity.48
After Percutaneous Coronary Intervention
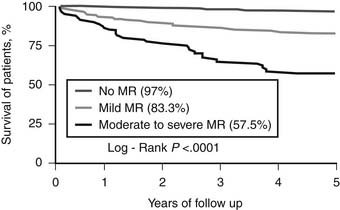
Pastorius and colleagues examined long-term outcomes in 711 patients who had moderate or greater mitral regurgitation at the time of percutaneous coronary intervention (PCI) and found that the presence of moderate or severe mitral regurgitation at that time was associated with a 5-year survival rate of 57% compared with 97% for those without regurgitation (Fig. 91-7).49 Even mild regurgitation was associated with reduced survival (5-year survival rate, 83%). These data corroborate the historical series from Ellis and colleagues,50 which demonstrated that patients with moderate to severe mitral regurgitation and ejection fraction below 40% had a 50% mortality at 3 years despite successful PCI. Other data from nonsurgical series also show that uncorrected regurgitation is associated with a poorer long-term survival.6 PCI should therefore not be considered definitive therapy for ischemic mitral regurgitation. The presence of mitral regurgitation should be an indication for surgical rather than percutaneous revascularization, provided the surgeon also addresses the mitral insufficiency.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree