ISCHEMIC HEART DISEASE
Ischemic heart disease (IHD) is a condition in which there is an inadequate supply of blood and oxygen to a portion of the myocardium; it typically occurs when there is an imbalance between myocardial oxygen supply and demand. The most common cause of myocardial ischemia is atherosclerotic disease of an epicardial coronary artery (or arteries) sufficient to cause a regional reduction in myocardial blood flow and inadequate perfusion of the myocardium supplied by the involved coronary artery. Chapter 30 deals with the development and treatment of atherosclerosis. This chapter focuses on the chronic manifestations and treatment of ischemic heart disease. The following chapters address the acute phases of this disease.
EPIDEMIOLOGY
 IHD causes more deaths and disability and incurs greater economic costs than any other illness in the developed world. IHD is the most common, serious, chronic, life-threatening illness in the United States, where 13 million persons have IHD, >6 million have angina pectoris, and >7 million have sustained a myocardial infarction. Genetic factors, a high-fat and energy-rich diet, smoking, and a sedentary lifestyle are associated with the emergence of IHD (Chap. 30). In the United States and Western Europe, it is growing among low-income groups, but primary prevention has delayed the disease to later in life in all socioeconomic groups. Despite these sobering statistics, it is worth noting that epidemiologic data show a decline in the rate of deaths due to IHD, about half of which is attributable to treatments and half to prevention by risk factor modification.
IHD causes more deaths and disability and incurs greater economic costs than any other illness in the developed world. IHD is the most common, serious, chronic, life-threatening illness in the United States, where 13 million persons have IHD, >6 million have angina pectoris, and >7 million have sustained a myocardial infarction. Genetic factors, a high-fat and energy-rich diet, smoking, and a sedentary lifestyle are associated with the emergence of IHD (Chap. 30). In the United States and Western Europe, it is growing among low-income groups, but primary prevention has delayed the disease to later in life in all socioeconomic groups. Despite these sobering statistics, it is worth noting that epidemiologic data show a decline in the rate of deaths due to IHD, about half of which is attributable to treatments and half to prevention by risk factor modification.
Obesity, insulin resistance, and type 2 diabetes mellitus are increasing and are powerful risk factors for IHD. With urbanization in countries with emerging economies and a growing middle class, elements of the energy-rich Western diet are being adopted. As a result, the prevalence of risk factors for and of IHD itself are both increasing rapidly in those regions such that a majority of the global burden of IHD occurs there. Population subgroups that appear to be particularly affected are men in South Asian countries, especially India and the Middle East. In light of the projection of large increases in IHD throughout the world, IHD is likely to become the most common cause of death worldwide by 2020.
PATHOPHYSIOLOGY
Central to an understanding of the pathophysiology of myocardial ischemia is the concept of myocardial supply and demand. In normal conditions, for any given level of a demand for oxygen, the myocardium will control the supply of oxygen-rich blood to prevent underperfusion of myocytes and the subsequent development of ischemia and infarction. The major determinants of myocardial oxygen demand (MVO2) are heart rate, myocardial contractility, and myocardial wall tension (stress). An adequate supply of oxygen to the myocardium requires a satisfactory level of oxygen-carrying capacity of the blood (determined by the inspired level of oxygen, pulmonary function, and hemoglobin concentration and function) and an adequate level of coronary blood flow. Blood flows through the coronary arteries in a phasic fashion, with the majority occurring during diastole. About 75% of the total coronary resistance to flow occurs across three sets of arteries: (1) large epicardial arteries (Resistance 1 = R1), (2) prearteriolar vessels (R2), and (3) arteriolar and intramyocardial capillary vessels (R3). In the absence of significant flow-limiting atherosclerotic obstructions, R1 is trivial; the major determinant of coronary resistance is found in R2 and R3.
The normal coronary circulation is dominated and controlled by the heart’s requirements for oxygen. This need is met by the ability of the coronary vascular bed to vary its resistance (and, therefore, blood flow) considerably while the myocardium extracts a high and relatively fixed percentage of oxygen. Normally, intramyocardial resistance vessels demonstrate an immense capacity for dilation (R2 and R3 decrease). For example, the changing oxygen needs of the heart with exercise and emotional stress affect coronary vascular resistance and in this manner regulate the supply of oxygen and substrate to the myocardium (metabolic regulation). The coronary resistance vessels also adapt to physiologic alterations in blood pressure to maintain coronary blood flow at levels appropriate to myocardial needs (autoregulation).
By reducing the lumen of the coronary arteries, atherosclerosis limits appropriate increases in perfusion when the demand for flow is augmented, as occurs during exertion or excitement. When the luminal reduction is severe, myocardial perfusion in the basal state is reduced. Coronary blood flow also can be limited by spasm (see Prinzmetal’s angina in Chap. 34), arterial thrombi, and, rarely, coronary emboli as well as by ostial narrowing due to aortitis. Congenital abnormalities such as the origin of the left anterior descending coronary artery from the pulmonary artery may cause myocardial ischemia and infarction in infancy, but this cause is very rare in adults.
Myocardial ischemia also can occur if myocardial oxygen demands are markedly increased and particularly when coronary blood flow may be limited, as occurs in severe left ventricular hypertrophy due to aortic stenosis. The latter can present with angina that is indistinguishable from that caused by coronary atherosclerosis largely owing to subendocardial ischemia (Chap. 20). A reduction in the oxygen-carrying capacity of the blood, as in extremely severe anemia or in the presence of carboxyhemoglobin, rarely causes myocardial ischemia by itself but may lower the threshold for ischemia in patients with moderate coronary obstruction.
Not infrequently, two or more causes of ischemia coexist in a patient, such as an increase in oxygen demand due to left ventricular hypertrophy secondary to hypertension and a reduction in oxygen supply secondary to coronary atherosclerosis and anemia. Abnormal constriction or failure of normal dilation of the coronary resistance vessels also can cause ischemia. When it causes angina, this condition is referred to as microvascular angina.
CORONARY ATHEROSCLEROSIS
Epicardial coronary arteries are the major site of atherosclerotic disease. The major risk factors for atherosclerosis (high levels of plasma low-density lipoprotein [LDL], low plasma high-density lipoprotein [HDL], cigarette smoking, hypertension, and diabetes mellitus [Chap. 30]) disturb the normal functions of the vascular endothelium. These functions include local control of vascular tone, maintenance of an antithrombotic surface, and control of inflammatory cell adhesion and diapedesis. The loss of these defenses leads to inappropriate constriction, luminal thrombus formation, and abnormal interactions between blood cells, especially monocytes and platelets, and the activated vascular endothelium. Functional changes in the vascular milieu ultimately result in the subintimal collections of fat, smooth-muscle cells, fibroblasts, and intercellular matrix that define the atherosclerotic plaque. This process develops at irregular rates in different segments of the epicardial coronary tree and leads eventually to segmental reductions in cross-sectional area, i.e., plaque formation.
There is also a predilection for atherosclerotic plaques to develop at sites of increased turbulence in coronary flow, such as at branch points in the epicardial arteries. When a stenosis reduces the diameter of an epicardial artery by 50%, there is a limitation of the ability to increase flow to meet increased myocardial demand. When the diameter is reduced by ~80%, blood flow at rest may be reduced, and further minor decreases in the stenotic orifice area can reduce coronary flow dramatically to cause myocardial ischemia at rest or with minimal stress.
Segmental atherosclerotic narrowing of epicardial coronary arteries is caused most commonly by the formation of a plaque, which is subject to rupture or erosion of the cap separating the plaque from the bloodstream. Upon exposure of the plaque contents to blood, two important and interrelated processes are set in motion: (1) platelets are activated and aggregate, and (2) the coagulation cascade is activated, leading to deposition of fibrin strands. A thrombus composed of platelet aggregates and fibrin strands traps red blood cells and can reduce coronary blood flow, leading to the clinical manifestations of myocardial ischemia.
The location of the obstruction influences the quantity of myocardium rendered ischemic and determines the severity of the clinical manifestations. Thus, critical obstructions in vessels, such as the left main coronary artery and the proximal left anterior descending coronary artery, are particularly hazardous. Chronic severe coronary narrowing and myocardial ischemia frequently are accompanied by the development of collateral vessels, especially when the narrowing develops gradually. When well developed, such vessels can by themselves provide sufficient blood flow to sustain the viability of the myocardium at rest but not during conditions of increased demand.
With progressive worsening of a stenosis in a proximal epicardial artery, the distal resistance vessels (when they function normally) dilate to reduce vascular resistance and maintain coronary blood flow. A pressure gradient develops across the proximal stenosis, and poststenotic pressure falls. When the resistance vessels are maximally dilated, myocardial blood flow becomes dependent on the pressure in the coronary artery distal to the obstruction. In these circumstances, ischemia, manifest clinically by angina or electrocardiographically by ST-segment deviation, can be precipitated by increases in myocardial oxygen demand caused by physical activity, emotional stress, and/or tachycardia. Changes in the caliber of the stenosed coronary artery due to physiologic vasomotion, loss of endothelial control of dilation (as occurs in atherosclerosis), pathologic spasm (Prinzmetal’s angina), or small platelet-rich plugs also can upset the critical balance between oxygen supply and demand and thereby precipitate myocardial ischemia.
EFFECTS OF ISCHEMIA
During episodes of inadequate perfusion caused by coronary atherosclerosis, myocardial tissue oxygen tension falls and may cause transient disturbances of the mechanical, biochemical, and electrical functions of the myocardium. Coronary atherosclerosis is a focal process that usually causes nonuniform ischemia. During ischemia, regional disturbances of ventricular contractility cause segmental hypokinesia, akinesia, or, in severe cases, bulging (dyskinesia), which can reduce myocardial pump function.
The abrupt development of severe ischemia, as occurs with total or subtotal coronary occlusion, is associated with almost instantaneous failure of normal muscle relaxation and then contraction. The relatively poor perfusion of the subendocardium causes more intense ischemia of this portion of the wall (compared with the subepicardial region). Ischemia of large portions of the ventricle causes transient left ventricular failure, and if the papillary muscle apparatus is involved, mitral regurgitation can occur. When ischemia is transient, it may be associated with angina pectoris; when it is prolonged, it can lead to myocardial necrosis and scarring with or without the clinical picture of acute myocardial infarction (Chap. 35).
A wide range of abnormalities in cell metabolism, function, and structure underlie these mechanical disturbances during ischemia. The normal myocardium metabolizes fatty acids and glucose to carbon dioxide and water. With severe oxygen deprivation, fatty acids cannot be oxidized, and glucose is converted to lactate; intracellular pH is reduced, as are the myocardial stores of high-energy phosphates, i.e., ATP and creatine phosphate. Impaired cell membrane function leads to the leakage of potassium and the uptake of sodium by myocytes as well as an increase in cytosolic calcium. The severity and duration of the imbalance between myocardial oxygen supply and demand determine whether the damage is reversible (≤20 min for total occlusion in the absence of collaterals) or permanent, with subsequent myocardial necrosis (>20 min).
Ischemia also causes characteristic changes in the electrocardiogram (ECG) such as repolarization abnormalities, as evidenced by inversion of T waves and, when more severe, displacement of ST segments (Chap. 11). Transient T-wave inversion probably reflects nontransmural, intramyocardial ischemia; transient ST-segment depression often reflects patchy subendocardial ischemia; and ST-segment elevation is thought to be caused by more severe transmural ischemia. Another important consequence of myocardial ischemia is electrical instability, which may lead to isolated ventricular premature beats or even ventricular tachycardia or ventricular fibrillation (Chap. 16). Most patients who die suddenly from IHD do so as a result of ischemia-induced ventricular tachyarrhythmias (Chap. 29).
ASYMPTOMATIC VERSUS SYMPTOMATIC IHD
Postmortem studies of accident victims and military casualties in Western countries have shown that coronary atherosclerosis often begins to develop before age 20 and is widespread even among adults who were asymptomatic during life. Exercise stress tests in asymptomatic persons may show evidence of silent myocardial ischemia, i.e., exercise-induced ECG changes not accompanied by angina pectoris; coronary angiographic studies of such persons may reveal coronary artery plaques and previously unrecognized obstructions (Chap. 13). Postmortem examination of patients with such obstructions without a history of clinical manifestations of myocardial ischemia often shows macroscopic scars secondary to myocardial infarction in regions supplied by diseased coronary arteries, with or without collateral circulation. According to population studies, ~25% of patients who survive acute myocardial infarction may not come to medical attention, and these patients have the same adverse prognosis as do those who present with the classic clinical picture of acute myocardial infarction (Chap. 35). Sudden death may be unheralded and is a common presenting manifestation of IHD (Chap. 29).
Patients with IHD also can present with cardiomegaly and heart failure secondary to ischemic damage of the left ventricular myocardium that may have caused no symptoms before the development of heart failure; this condition is referred to as ischemic cardiomyopathy. In contrast to the asymptomatic phase of IHD, the symptomatic phase is characterized by chest discomfort due to either angina pectoris or acute myocardial infarction (Chap. 35). Having entered the symptomatic phase, the patient may exhibit a stable or progressive course, revert to the asymptomatic stage, or die suddenly.
STABLE ANGINA PECTORIS
This episodic clinical syndrome is due to transient myocardial ischemia. Various diseases that cause myocardial ischemia as well as the numerous forms of discomfort with which it may be confused are discussed in Chap. 4. Males constitute ~70% of all patients with angina pectoris and an even greater proportion of those less than 50 years of age. It is, however, important to note that angina pectoris in women is often atypical in presentation (see later).
HISTORY
The typical patient with angina is a man >50 years or a woman >60 years of age who complains of episodes of chest discomfort, usually described as heaviness, pressure, squeezing, smothering, or choking and only rarely as frank pain. When the patient is asked to localize the sensation, he or she typically places a hand over the sternum, sometimes with a clenched fist, to indicate a squeezing, central, substernal discomfort (Levine’s sign). Angina is usually crescendo-decrescendo in nature, typically lasts 2 to 5 min, and can radiate to either shoulder and to both arms (especially the ulnar surfaces of the forearm and hand). It also can arise in or radiate to the back, interscapular region, root of the neck, jaw, teeth, and epigastrium. Angina is rarely localized below the umbilicus or above the mandible. A useful finding in assessing a patient with chest discomfort is the fact that myocardial ischemic discomfort does not radiate to the trapezius muscles; that radiation pattern is more typical of pericarditis.
Although episodes of angina typically are caused by exertion (e.g., exercise, hurrying, or sexual activity) or emotion (e.g., stress, anger, fright, or frustration) and are relieved by rest, they also may occur at rest (see “Unstable Angina Pectoris,” [Chap. 34]) and while the patient is recumbent (angina decubitus). The patient may be awakened at night by typical chest discomfort and dyspnea. Nocturnal angina may be due to episodic tachycardia, diminished oxygenation as the respiratory pattern changes during sleep, or expansion of the intrathoracic blood volume that occurs with recumbency; the latter causes an increase in cardiac size (end-diastolic volume), wall tension, and myocardial oxygen demand that can lead to ischemia and transient left ventricular failure.
The threshold for the development of angina pectoris may vary by time of day and emotional state. Many patients report a fixed threshold for angina, which occurs predictably at a certain level of activity, such as climbing two flights of stairs at a normal pace. In these patients, coronary stenosis and myocardial oxygen supply are fixed, and ischemia is precipitated by an increase in myocardial oxygen demand; they are said to have stable exertional angina. In other patients, the threshold for angina may vary considerably within any particular day and from day to day. In such patients, variations in myocardial oxygen supply, most likely due to changes in coronary vasomotor tone, may play an important role in defining the pattern of angina. A patient may report symptoms upon minor exertion in the morning (a short walk or shaving) yet by midday be capable of much greater effort without symptoms. Angina may also be precipitated by unfamiliar tasks, a heavy meal, exposure to cold, or a combination of these factors.
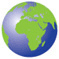
Exertional angina typically is relieved in 1 to 5 min by slowing or ceasing activities and even more rapidly by rest and sublingual nitroglycerin (see later). Indeed, the diagnosis of angina should be suspect if it does not respond to the combination of these measures. The severity of angina can be conveniently summarized by the Canadian Cardiac Society functional classification (Table 33-1). Its impact on the patient’s functional capacity can be described by using the New York Heart Association functional classification (Table 33-1).
TABLE 33-1
CARDIOVASCULAR DISEASE CLASSIFICATION CHART
Sharp, fleeting chest pain or a prolonged, dull ache localized to the left submammary area is rarely due to myocardial ischemia. However, especially in women and diabetic patients, angina pectoris may be atypical in location and not strictly related to provoking factors. In addition, this symptom may exacerbate and remit over days, weeks, or months. Its occurrence can be seasonal, occurring more frequently in the winter in temperate climates. Anginal “equivalents” are symptoms of myocardial ischemia other than angina. They include dyspnea, nausea, fatigue, and faintness and are more common in the elderly and in diabetic patients.
Systematic questioning of a patient with suspected IHD is important to uncover the features of an unstable syndrome associated with increased risk, such as angina occurring with less exertion than in the past, occurring at rest, or awakening the patient from sleep. Since coronary atherosclerosis often is accompanied by similar lesions in other arteries, a patient with angina should be questioned and examined for peripheral arterial disease (intermittent claudication [Chap. 39]), stroke, or transient ischemic attacks. It is also important to uncover a family history of premature IHD (<55 years in first-degree male relatives and <65 in female relatives) and the presence of diabetes mellitus, hyperlipidemia, hypertension, cigarette smoking, and other risk factors for coronary atherosclerosis (Chap. 30).
The history of typical angina pectoris establishes the diagnosis of IHD until proven otherwise. In patients with atypical angina (Chap. 4), the coexistence of advanced age, male sex, the postmenopausal state, and risk factors for atherosclerosis increase the likelihood of hemodynamically significant coronary disease. A particularly challenging problem is the evaluation and management of patients with persistent ischemic-type chest discomfort but no flow-limiting obstructions in their epicardial coronary arteries. This situation arises more often in women than in men. Potential etiologies include microvascular coronary disease (detectable on coronary reactivity testing in response to vasoactive agents such as intracoronary adenosine, acetylcholine, and nitroglycerin) and abnormal cardiac nociception. Treatment of microvascular coronary disease should focus on efforts to improve endothelial function, including nitrates, beta blockers, calcium antagonists, statins, and angiotensin-converting enzyme (ACE) inhibitors. Abnormal cardiac nociception is more difficult to manage and may be ameliorated in some cases by imipramine.
PHYSICAL EXAMINATION
The physical examination is often normal in patients with stable angina when they are asymptomatic. However, because of the increased likelihood of ischemic heart disease in patients with diabetes and/or peripheral arterial disease, clinicians should search for evidence of atherosclerotic disease at other sites, such as an abdominal aortic aneurysm, carotid arterial bruits, and diminished arterial pulses in the lower extremities. The physical examination also should include a search for evidence of risk factors for atherosclerosis such as xanthelasmas and xanthomas (Chap. 30). Evidence for peripheral arterial disease should be sought by evaluating the pulse contour at multiple locations and comparing the blood pressure between the arms and between the arms and the legs (ankle-brachial index). Examination of the fundi may reveal an increased light reflex and arteriovenous nicking as evidence of hypertension. There also may be signs of anemia, thyroid disease, and nicotine stains on the fingertips from cigarette smoking.
Palpation may reveal cardiac enlargement and abnormal contraction of the cardiac impulse (left ventricular dyskinesia). Auscultation can uncover arterial bruits, a third and/or fourth heart sound, and, if acute ischemia or previous infarction has impaired papillary muscle function, an apical systolic murmur due to mitral regurgitation. These auscultatory signs are best appreciated with the patient in the left lateral decubitus position. Aortic stenosis, aortic regurgitation (Chap. 20), pulmonary hypertension (Chap. 40), and hypertrophic cardiomyopathy (Chap. 21) must be excluded, since these disorders may cause angina in the absence of coronary atherosclerosis. Examination during an anginal attack is useful, since ischemia can cause transient left ventricular failure with the appearance of a third and/or fourth heart sound, a dyskinetic cardiac apex, mitral regurgitation, and even pulmonary edema. Tenderness of the chest wall, localization of the discomfort with a single fingertip on the chest, or reproduction of the pain with palpation of the chest makes it unlikely that the pain is caused by myocardial ischemia. A protuberant abdomen may indicate that the patient has the metabolic syndrome and is at increased risk for atherosclerosis.
LABORATORY EXAMINATION
Although the diagnosis of IHD can be made with a high degree of confidence from the history and physical examination, a number of simple laboratory tests can be helpful. The urine should be examined for evidence of diabetes mellitus and renal disease (including microalbuminuria) since these conditions accelerate atherosclerosis. Similarly, examination of the blood should include measurements of lipids (cholesterol—total, LDL, HDL—and triglycerides), glucose (hemoglobin A1C), creatinine, hematocrit, and, if indicated based on the physical examination, thyroid function. A chest x-ray is important as it may show the consequences of IHD, i.e., cardiac enlargement, ventricular aneurysm, or signs of heart failure. These signs can support the diagnosis of IHD and are important in assessing the degree of cardiac damage. Evidence exists that an elevated level of high-sensitivity C-reactive protein (CRP) (specifically, between 0 and 3 mg/dL) is an independent risk factor for IHD and may be useful in therapeutic decision making about the initiation of hypolipidemic treatment. The major benefit of high-sensitivity CRP is in reclassifying the risk of IHD in patients in the “intermediate” risk category on the basis of traditional risk factors.
ELECTROCARDIOGRAM
A 12-lead ECG recorded at rest may be normal in patients with typical angina pectoris, but there may also be signs of an old myocardial infarction (Chap. 11). Although repolarization abnormalities, i.e., ST-segment and T-wave changes, as well as left ventricular hypertrophy and disturbances of cardiac rhythm or intraventricular conduction are suggestive of IHD, they are nonspecific, since they also can occur in pericardial, myocardial, and valvular heart disease or, in the case of the former, transiently with anxiety, changes in posture, drugs, or esophageal disease. The presence of left ventricular hypertrophy (LVH) is a significant indication of increased risk of adverse outcomes from ischemic heart disease. Of note, even though LVH and cardiac rhythm disturbances are nonspecific indicators of the development of IHD, they may be contributing factors to episodes of angina in patients in whom IHD has developed as a consequence of conventional risk factors. Dynamic ST-segment and T-wave changes that accompany episodes of angina pectoris and disappear thereafter are more specific.
STRESS TESTING
Electrocardiographic
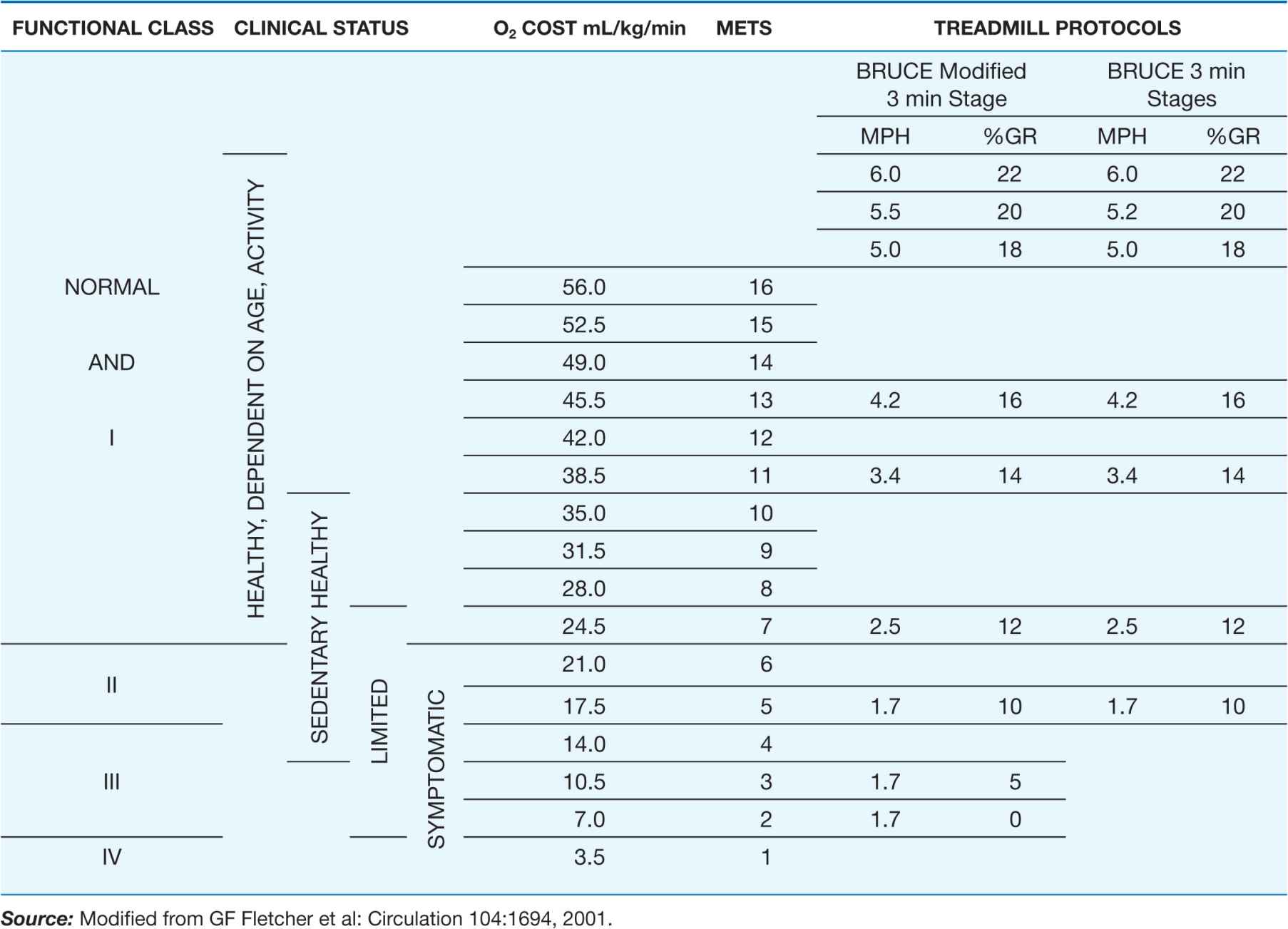
The most widely used test for both the diagnosis of IHD and the estimation of risk and prognosis involves recording the 12-lead ECG before, during, and after exercise, usually on a treadmill (Fig. 33-1). The test consists of a standardized incremental increase in external workload (Table 33-2) while symptoms, the ECG, and arm blood pressure are monitored. Exercise duration is usually symptom-limited, and the test is discontinued upon evidence of chest discomfort, severe shortness of breath, dizziness, severe fatigue, ST-segment depression >0.2 mV (2 mm), a fall in systolic blood pressure >10 mmHg, or the development of a ventricular tachyarrhythmia. This test is used to discover any limitation in exercise performance, detect typical ECG signs of myocardial ischemia, and establish their relationship to chest discomfort. The ischemic ST-segment response generally is defined as a flat or downsloping depression of the ST segment >0.1 mV below baseline (i.e., the PR segment) and lasting longer than 0.08 s (Fig. 33-1). Upsloping or junctional ST-segment changes are not considered characteristic of ischemia and do not constitute a positive test. Although T-wave abnormalities, conduction disturbances, and ventricular arrhythmias that develop during exercise should be noted, they are also not diagnostic. Negative exercise tests in which the target heart rate (85% of maximal predicted heart rate for age and sex) is not achieved are considered nondiagnostic.
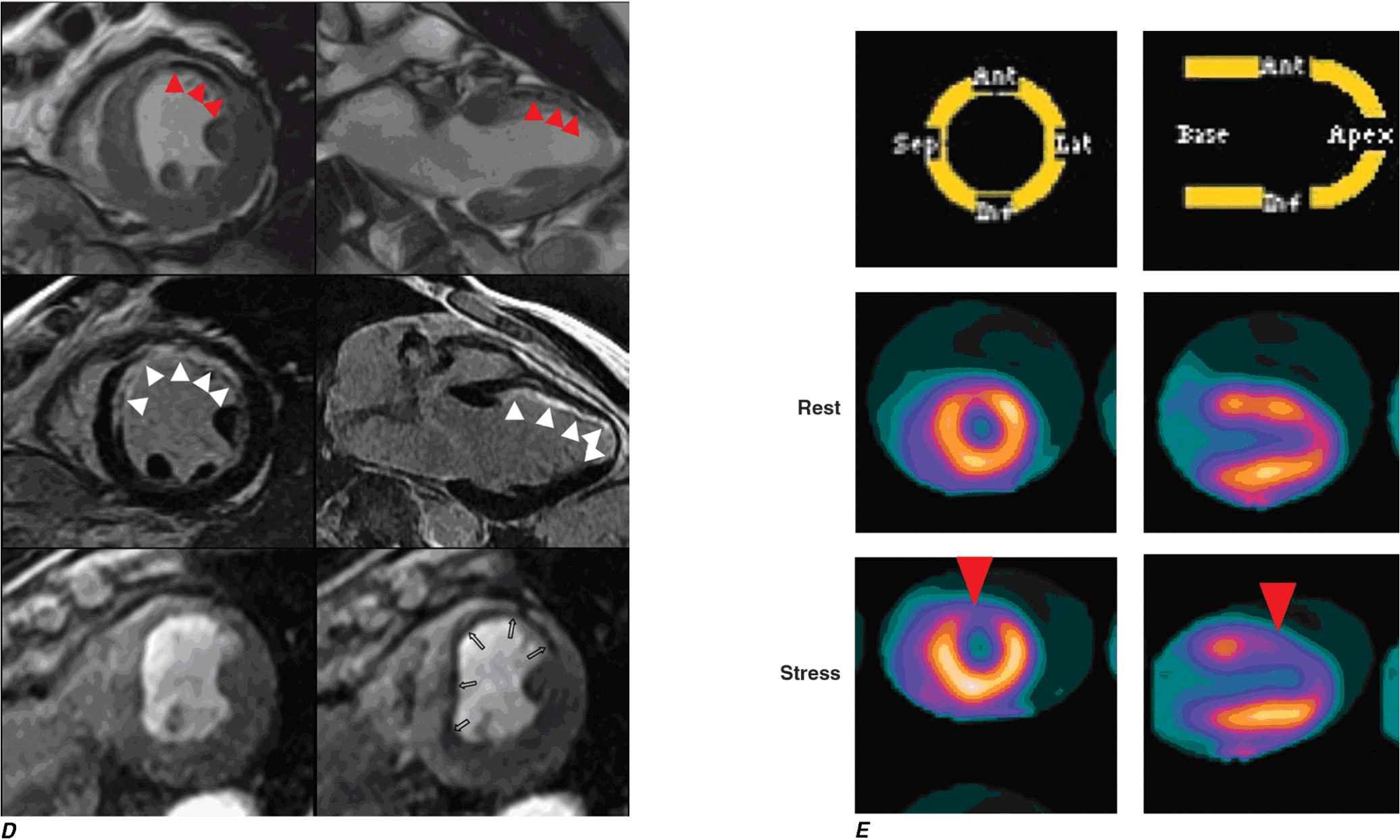
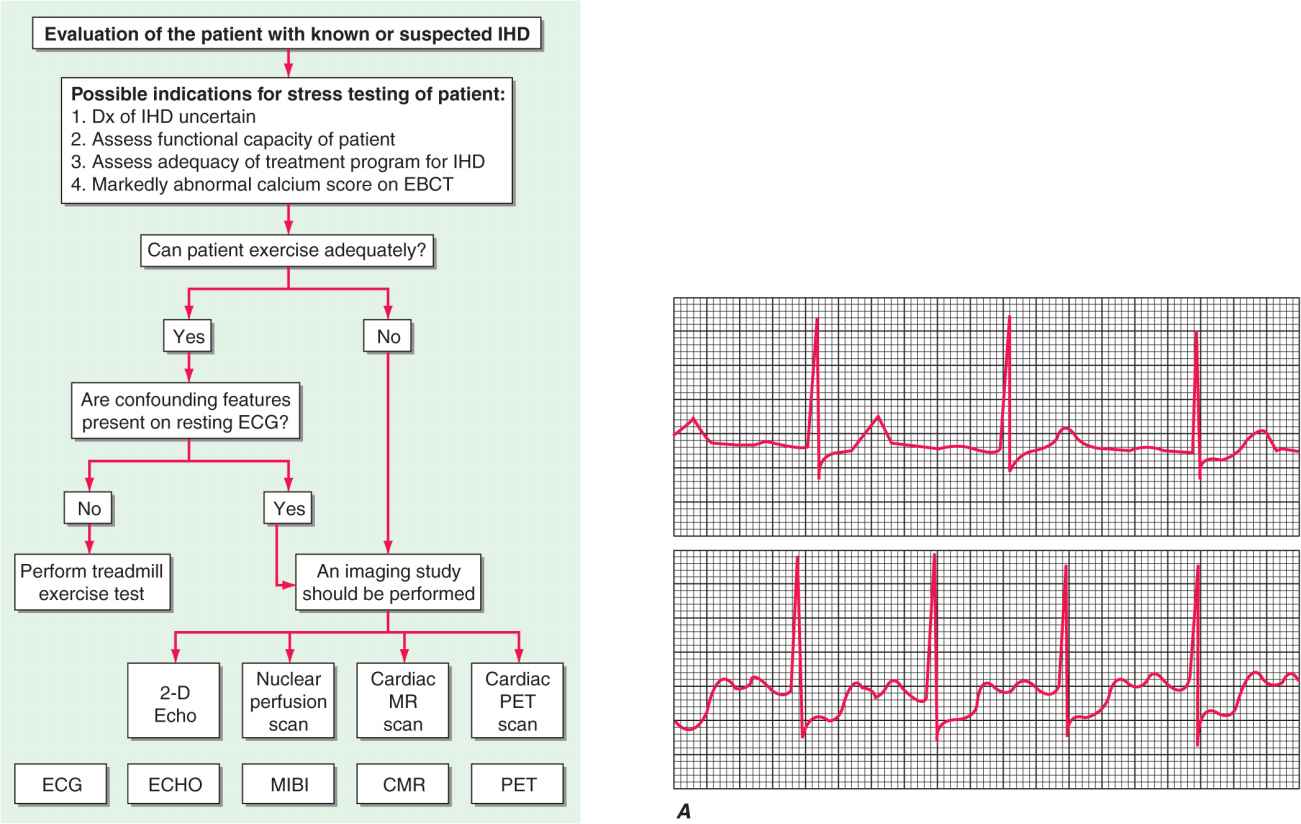
FIGURE 33-1
Evaluation of the patient with known or suspected ischemic heart disease. At the top of the figure is an algorithm for identifying patients who should be referred for stress testing and the decision pathway for determining whether a standard treadmill exercise with ECG monitoring alone is adequate. A specialized imaging study is necessary if the patient cannot exercise adequately (pharmacologic challenge is given) or if there are confounding features on the resting ECG (symptom-limited treadmill exercise may be used to stress the coronary circulation). At the bottom of the figure are examples of the data obtained with ECG monitoring and specialized imaging procedures. CMR, cardiac magnetic resonance; EBCT, electron beam computed tomography; ECG, electrocardiogram; ECHO, echocardiography; IHD, ischemic heart disease; MIBI, methoxyisobutyl isonitrite; MR, magnetic resonance; PET, positron emission tomography.
A. Lead V4 at rest (top) and after 4½ min of exercise (bottom). There is 3 mm (0.3 mV) of horizontal ST-segment depression, indicating a positive test for ischemia. (Modified from BR Chaitman, in E Braunwald et al [eds]: Heart Disease, 6th ed, Philadelphia, Saunders, 2001.)
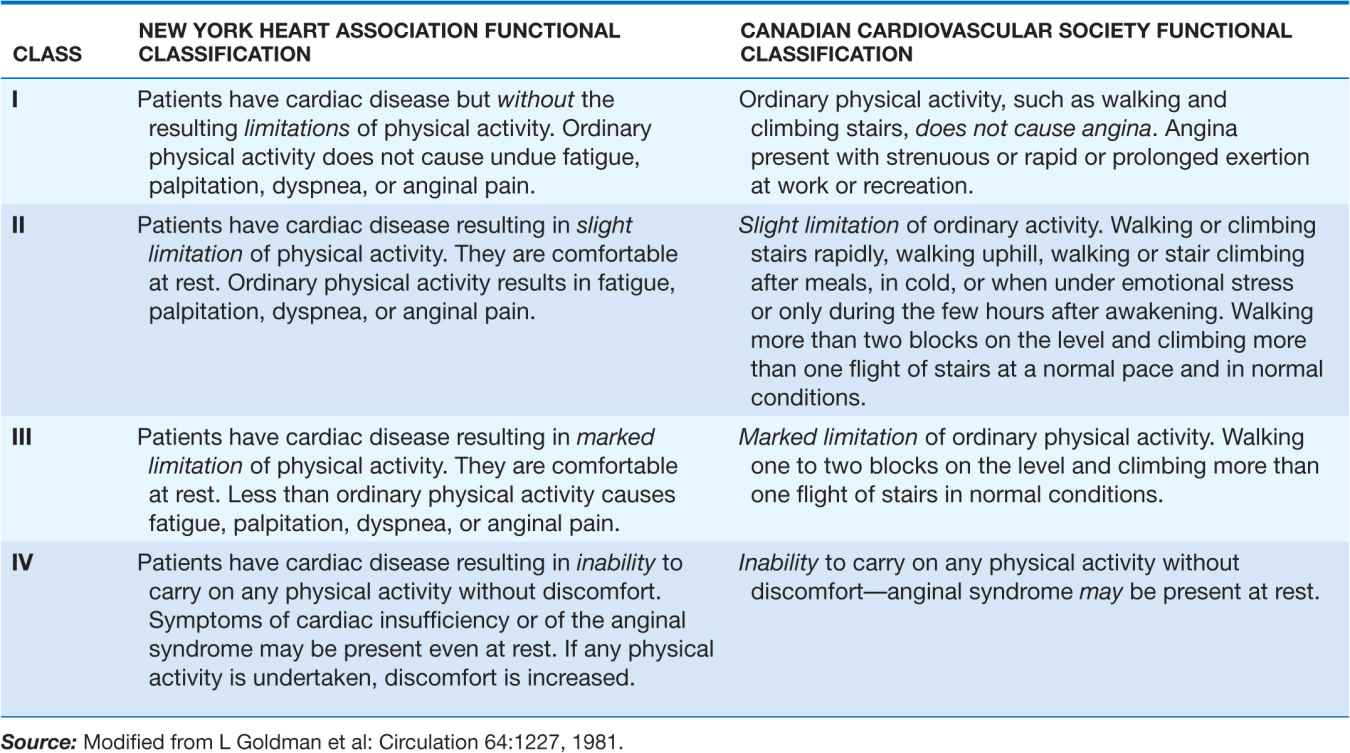
B. 45-year-old avid jogger who began experiencing classic substernal chest pressure underwent an exercise echo study. With exercise the patient’s heart rate increased from 52 to 153 bpm. The left ventricular chamber dilated with exercise, and the septal and apical portions became akinetic to dyskinetic (red arrow). These findings are strongly suggestive of a significant flow-limiting stenosis in the proximal left anterior descending artery, which was confirmed at coronary angiography. (Modified from SD Solomon, in E. Braunwald et al [eds]: Primary Cardiology, 2nd ed, Philadelphia, Saunders, 2003.)
C. Stress and rest myocardial perfusion SPECT images obtained with 99m-technetium sestamibi in a patient with chest pain and dyspnea on exertion. The images demonstrate a medium-size and severe stress perfusion defect involving the inferolateral and basal inferior walls, showing nearly complete reversibility, consistent with moderate ischemia in the right coronary artery territory (red arrows). (Images provided by Dr. Marcello Di Carli, Nuclear Medicine Division, Brigham and Women’s Hospital, Boston, MA.)
D. A patient with a prior myocardial infarction presented with recurrent chest discomfort. On cardiac magnetic resonance (CMR) cine imaging, a large area of anterior akinesia was noted (marked by the arrows in the top left and right images, systolic frame only). This area of akinesia was matched by a larger extent of late gadolinium-DTPA enhancements consistent with a large transmural myocardial infarction (marked by arrows in the middle left and right images). Resting (bottom left) and adenosine vasodilating stress (bottom right) first-pass perfusion images revealed reversible perfusion abnormality that extended to the inferior septum. This patient was found to have an occluded proximal left anterior descending coronary artery with extensive collateral formation. This case illustrates the utility of different modalities in a CMR examination in characterizing ischemic and infarcted myocardium. DTPA, diethylenetriamine penta-acetic acid. (Images provided by Dr. Raymond Kwong, Cardiovascular Division, Brigham and Women’s Hospital, Boston, MA.)
E. Stress and rest myocardial perfusion PET images obtained with rubidium-82 in a patient with chest pain on exertion. The images demonstrate a large and severe stress perfusion defect involving the mid and apical anterior, anterolateral, and anteroseptal walls and the LV apex, showing complete reversibility, consistent with extensive and severe ischemia in the mid-left anterior descending coronary artery territory (red arrows). (Images provided by Dr. Marcello Di Carli, Nuclear Medicine Division, Brigham and Women’s Hospital, Boston, MA.)
TABLE 33-2
RELATION OF METABOLIC EQUIVALENT TASKS (METs) TO STAGES IN VARIOUS TESTING PROTOCOLS
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree