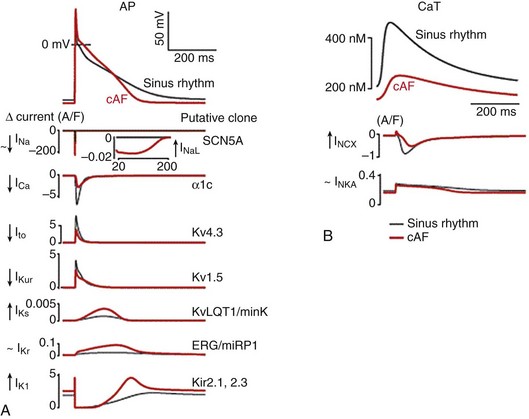
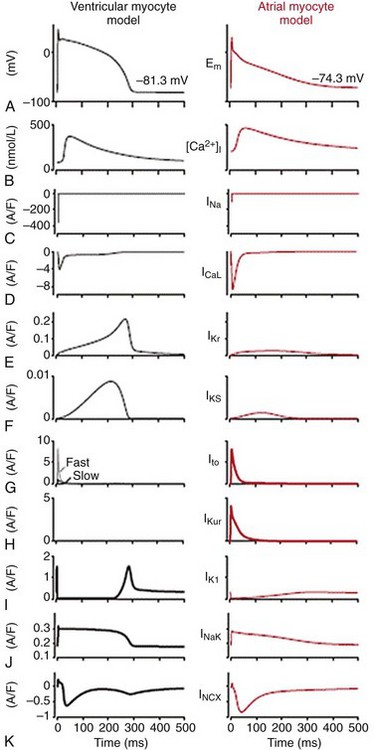
31 Our knowledge regarding the ionic bases of the atrial action potential has evolved continuously since the early transmembrane recordings made in isolated human atrial tissue almost 50 years ago.1,2 The advent of the patch-clamp technique in the early 1980s3 and the subsequent detailed characterization of the biophysical properties of the various ionic currents in human atrial cells4–7 led to the development of the first detailed quantitative mathematical models of the human atrial action potential in 1998.8,9 This was followed by increased recognition that intracellular Ca2+ ([Ca2+]i) homeostasis plays a major role in influencing atrial repolarization in both normal and pathophysiological conditions, particularly in atrial fibrillation (AF).10–16 It resulted in formulations of newer quantitative models in 2011 that encapsulate the nonlinear interactions between ionic currents and (Ca2+)i to simulate human atrial excitation-contraction (E-C) coupling.17,18 Studies have also documented regional variations in atrial electrophysiology,19 the influences of age-related changes,20 and the ionic bases of atrial remodeling that occur as the result of AF21 and/or ventricular dysfunction.22 This chapter provides an overview of the principal ionic determinants of atrial action potentials at the cellular level in health and disease (mainly humans), their regional heterogeneities and age-related changes, and their behavior during functional reentry caused by one or more spiral waves (rotors) that have now been shown to sustain AF in animal models23 and more recently in humans.24,25 We recently developed a mathematical model of human atrial cell electrophysiology17 based on available experimental data. The atrial formulation was derived from an existing model of the human ventricular cell.26 By incorporating experimentally known ionic differences between atrial and ventricular myocytes, the model reconstructs essential aspects of atrial E-C coupling. It allows a systematic comparison of the main ionic currents underlying the human atrial action potential (Figure 31-1, in red) and highlights key differences with those underlying the ventricular action potential (Figure 31-1, in black). Figure 31-1 Key ionic currents underlying ventricular (black) and atrial (red) action potentials. Depicted in this figure are (A) action potentials, (B) Ca2+ transients ([Ca2+]i), (C) sodium current, INa, (D) calcium current, ICaL, (E) rapid delayed rectifier current, IKr, (F) slow delayed rectifier current, IKs, (G) transient outward current, Ito, (H) ultra-rapid delayed rectifier current, IKur, (I) inward rectifier current, IK1, (J) Na+/K+ pump current, INaK, and (K) Na+/Ca2+ exchanger current, INCX. The human atrial action potential typically demonstrates a triangular morphology (compared with a spike-and-dome shape with a prominent plateau phase of the ventricular counterpart).1,27 The human atrial action potential duration at 90% repolarization (APD90) at 1 Hz shows large variations of between 150 and 500 msec, possibly influenced by recording conditions and ionic concentrations.2,4,27–31 The atrial resting membrane potential (Vrest) has been found to vary between −65 and −80 mV,2,4,27–31 and is more depolarized than in the human ventricle26,27 (also see Figure 31-1). The depolarized Vrest in atrial cells compared with ventricular cells is mainly attributed to differences in density of the inward rectifier K+ current, IK117,32,33 (also see later). Maximum upstroke velocities (Vmax) for atrial action potentials have been experimentally reported to vary between ≈150 and 300 V/s,2,27–31 in contrast to higher values of 300 to 400 V/s for human ventricular cells.26,33 Characteristic human atrioventricular action potential property/shape differences are replicated in most mammalian species,19 except for the murine myocardium (rat/mouse), where both atrial and ventricular cells display short, triangular action potentials.34,35 Initiation of the atrial action potential is due to rapid activation of the inward Na+ current, INa, which is also the principal determinant of Vmax. Functional differences in human ventricular and atrial INa biophysical properties were reported to be minimal,36–39 but recent studies have shown that the molecular correlates of INa in the human atrial and ventricular myocardium are different. Although Nav1.5 was found to be the main α-subunit encoding for atrial/ventricular INa, the transcript for the β-subunit, Navβ1, was more prominently expressed in the human atrium.40 In addition, recent reports in canine atrial cells suggest that atrial INa has higher current density and a more negative steady-state half-inactivation voltage value, when compared with ventricular cells.41 Similar differences were reported in guinea pig atrial and ventricular cells.42 Further, because of the more depolarized Vrest, the availability of atrial INa is less, which results in a smaller INa underlying the atrial action potential, compared with the ventricular counterpart, as can be seen in Figure 31-1. The L-type Ca2+ current (ICaL) is mainly responsible for the plateau phase of the atrial action potential, as well as the Ca2+-induced Ca2+ release in human atrial cells.43 Patch-clamp experiments suggested that the T-type Ca2+ current (ICaT) was not present in human atrial cells.43 Experimental studies conducted in the Lederer lab reported differences in the biophysical properties of ICaL between human atrial and ventricular cells in current density and steady-state inactivation properties.44 At the transcript level, greater expression of the α-subunits Cav1.3 and Cav3.1 has been reported in the atrium, compared with the ventricle.40 Moreover, a more “negative voltage” of the triangular plateau phase causes a larger driving force (Vm − ECa; where Vm represents membrane voltage, and ECa represents reversal potential for ICaL), resulting in an ICaL current with a larger magnitude underlying the human atrial cell action potential (compared with its ventricular counterpart), as can be seen in Figure 31-1.17 Differences in regulation of human atrial (but not ventricular) ICaL by second messengers such as serotonin45 and phosphodiesterases46 (PDEs) have been reported and may further influence action potential morphology.47 The key current that sets the Vrest (i.e., the inward rectifier K+ current IK1) has a much smaller density (≈5-6-fold less) in human atrial cells compared with its ventricular counterpart,17,32 and this partially explains the depolarized Vrest in atrial as opposed to ventricular cells (see Figure 31-1). The smaller density of IK1 is also responsible in part for the slower late phase of repolarization in the atrium. The main molecular correlate of IK1, Kir2.1, is more robustly expressed in the human ventricle than in the atrium.40 The human atrium also expresses the fast, depolarization-activated, 4-AP (aminopyridine)-sensitive, Ca2+-independent transient outward K+ current, Ito1.4,27 This current is responsible for the initial repolarization phase of the atrial action potential4,17,27,48,49 (see Figure 31-1). It is interesting to note that despite having almost a 2-fold higher density in the atrium than in the ventricle,27 the magnitude of Ito1 underlying the atrial and ventricular action potentials is similar, likely as the result of action potential morphologies (see Figure 31-1). The main molecular correlates of human atrial Ito1 are Kv4.3/KChiP2 (α/β-subunits, respectively).40 The slow component of Ito1,slow (mainly encoded by Kv1.4) is absent in the human atrium, but present in the ventricle.17,26 A Ca2+-dependent transient outward K+ current, Ito2, has been reported in human atrial cells, but both its molecular correlate and its contribution to the action potential remain unclear.48 Human atrial cells also express three functional delayed rectifier K+ currents, which contribute to repolarization: the ultra-rapid delayed rectifier K+ current, IKur (molecular correlate Kv1.5)6,50 and the rapid and slow delayed rectifier K+ currents, viz, IKr, and IKs (molecular correlates HERG, KvLQT1).7,51,52 IKur is present only in the atrium (not ventricle), is active during the plateau phase (see Figure 31-1), and is blocked by low concentrations of 4-AP (100 µM).5,6,50 Its atrial-selective nature has made it an attractive target for many antiarrhythmic drugs in development for terminating and/or preventing recurrence of AF.53 Compared with IKur, the contribution of IKr and IKs to the human atrial action potential is small (see Figure 31-1),17 in part because of their small density, and in part because of their triangular shape and plateau phase at relatively negative membrane voltages, which preclude both IKr and IKs from activating fully. However, considerable variability in the shape of the human atrial action potentials has been noted, and IKr/IKs are likely to contribute more in cells showing a prominent plateau phase and dome (the so-called type 1 action potentials).7 The human atrial action potential is also modulated by [Ca2+]i, which directly influences the inactivation of ICaL54 and the magnitude and temporal profile of the electrogenic Na+/Ca2+ exchanger current, INCX10,55 (molecular transcript NCX140), and has an indirect influence on the Na+/K+ pump current, INaK, by influencing intracellular Na+ ion accumulation17 (molecular transcripts Na/K ATPase α1, α3, β140). The Na+/Ca2+ exchanger current (INCX) is the main Ca2+ extrusion and Na+ influx pathway in cardiac myocytes. It extrudes 1 Ca+ in exchange for 3 Na+, thus generating an inward current that influences cardiac repolarization and arrhythmogenesis.56 The Na+/K+ pump (NKA) is the main route of Na+ efflux in cardiac cells, thus regulating intracellular [Na+]. By extruding 3 Na+ in exchange for 2 K+, it generates an outward current that is known to influence both resting membrane potential and repolarization.56 We recently simulated the [Ca2+]i homeostasis in human atrial cells, and our results showed that whereas INaK is primarily a repolarizing outward current, INCX can be both outward and inward; it contributes to repolarization in the early phase of the action potential, and is a negative current (depolarizing influence) during the later phase of the action potential, when it extrudes Ca2+ ions from the atrial cells (see Figure 31-1).17 INaK and INCX also influence the frequency dependence of the human atrial action potential duration, which is discussed in later sections. Besides the currents discussed earlier, other ion channels are activated under specific conditions and can influence the human atrial action potential. These include the acetylcholine-activated K+ current, IKACh28,57,58 (molecular correlates Kir3.1/3.440), the adenosine triphosphate (ATP)-sensitive K+ current, IKATP59,60 (molecular correlate, Kir6.1/6.2/SuR2.X40), the Ca2+-dependent nonselective cation current61,62 (molecular correlates unknown), and the hyperpolarization-activated funny current, If63,78,93 (molecular correlates HCN1/HCN440). Some reports indicate that a Ca2+-activated K+ current IKCa (putative molecular correlate SK240) may modulate the human atrial action potential64,65; however, its presence and its contribution to repolarization remain controversial, and species-specific variations have been suggested.66 Stretch-activated ion channels (ISAC), which have been reported in human atrial cells,67 influence both Vrest and APD; however, their molecular correlates remain unknown. Swelling-induced, outwardly rectifying chloride channels, such as ICl−68 (putative molecular correlates Cl3,Cl6,Cl740), have been reported in human atrial cells. The transcripts of two-pore channels (TWIK1, TASK1) have also been reported to be present in the human atrium.40 However, no information is available on the contribution of these currents or that of ICl− to the APD. The atrium is a complicated three-dimensional (3D) structure, and the heterogeneities in electrical properties between different regions, such as the left atrium (LA), the right atrium (RA), and the pulmonary veins (PV) and their ionic basis have been extensively studied in many species, including mouse,69,70 rabbit,71,72 and dog.73,74 Information regarding such heterogeneity in humans is limited, but recent studies have begun to address the putative ionic/molecular basis for these differences. The frequencies of excitation in AF have been reported to show regional gradients during paroxysmal AF and postoperative AF, with LA-PV regions displaying the highest frequencies.75–77 This is highly indicative of underlying differences in biophysical properties of some ionic currents, and these variations, which are more prominent in disease conditions, are discussed later. In patients in normal sinus rhythm, Caballero and colleagues reported a higher density of IKur in the right than in the left atrial cardiomyocytes.52 Incorporating such data in human atrial mathematical models did not result in appreciable differences between the LA/RA action potentials in normal sinus rhythm.17 However, large variations in human atrial action potential shapes (mainly triangular, some dome shaped) have been reported in cells isolated from the right atrial appendage, and this has been attributed to differences in current density ratios of Ito1/IKr in these cells.7 Recently, the carbachol-activated K+ current (IKACh) was reported to be 70% larger in RA than in LA, in sinus rhythm patients, with correspondingly higher protein expressions of its molecular correlates (Kir3.1/Kir3.4) in RA versus LA.79 To the best of our knowledge, no report has described the ionic mechanisms underlying human PV cells. Thus available data regarding the regional variations in human atrial electrophysiology/underlying ionic mechanisms under healthy conditions are very limited, and further studies are needed in this regard. In the next paragraph, we briefly review the atrial action potential heterogeneity/ionic mechanisms in other animal species. In healthy hearts of dogs and mice, the APD is shorter in LA than in RA, primarily on account of differences in current densities of the delayed rectifier K+ currents, that is, IKr in dogs and IKur and the steady-state K+ current Iss in mice display larger current densities in LA than in RA.70,80,81 In sheep atrial cells, the IKACh current has been shown to have a larger density in LA than in RA, whereas the density of IK1 was similar.82 In contrast, IKACh current density was larger in RA than in LA in mouse,83 similar to humans in sinus rhythm.79 The density of INa has been reported to be larger in LA than in RA in dogs.84 Besides LA and RA, differences within RA have also been reported. For example, the action potential properties of the pectinate muscles and the crista terminalis in the rabbit RA are different85; such differences have been attributed to differences in various ion channels (Na+, Ca2+, K+) and have been integrated into a mathematical model.86 Furthermore, differences in the electrophysiological properties of cells isolated from the LA and PV regions have been extensively studied by the Nattel group in dogs73,74 and by the Chen group in rabbits.71,72 Canine PV cells exhibit a depolarized Vrest, a smaller Vmax, and shorter APD compared with LA cells.73 This has been attributed to a greater density of IKr, IKs, and a smaller density of ICaL, Ito1 in PV than in LA cells, whereas the densities of INCX and ICaT were found to be similar between PV and LA cells.73 It is interesting to note that Ca2+-handling properties were not different between canine PV and LA cells.87 In rabbit PV cells, most cells demonstrated pacemaker activity.72 As reported in the ventricle, epi-endocardial differences also exist within the atrium. A recent study in pigs reported a shorter refractory period in the atrial epicardium compared with the endocardium.88 This is in line with a shorter atrial epicardial APD recorded in the canine right atrial free wall compared with the endocardium.89 The canine atrial endocardium was also more sensitive to APD shortening after the addition of acetylcholine.89 However, the ionic mechanisms underlying atrial epicardium-endocardium differences remain unexplored. Age-related changes in atrial action potential differences in humans have been most systematically investigated between infants (neonatal) and adults.29,30 Adult cells displayed a more prominent initial notch compared with neonatal atrial cells.29,30 The properties of Ito1 were significantly different as well; Ito1 displayed significantly higher density, faster inactivation, and a slower recovery from inactivation in neonatal compared with adult human atrial cells.29 Correspondingly, the protein density of Kv4.3 was higher and KChiP2 was lower in neonatal compared with adult cells.29 The properties of ICaL were also different: The basal density was smaller and the protein density of Giα3 was larger in neonatal compared with adult cells, resulting in a different response to a lower dosage of β-adrenergic stimulation.90 In addition, Ca2+ transients, which influence both adult and neonatal human atrial action potentials,14,17,91 may display inherently different biophysical properties but remain unexplored. It is well known that the propensity for AF increases with age,92 but very few studies have been conducted to explore potential differences between adult and aged human atrial electrophysiology, and most of the knowledge in this area has been derived from animal models (rat, rabbit, dog). This topic was reviewed comprehensively in a recent article. We briefly describe the salient points.20 Vrest was found to be more depolarized in rats/dogs,94,95 whereas APD90 was more prolonged in rats/rabbits/dogs in aged compared with adult atria.20,96,97 The density of INa was not different,84 but the density of ICaL was reported to be smaller in canine atrial cells.98 Further, the density of Ito1 was higher, its steady-state half-inactivation value showed more positive values, and recovery from inactivation was slower in canine aged atrial cells compared with their adult counterparts.98 The density of the sustained outward K+ current, Isus, was also reported to be larger in aged canine atrial cells.98 In addition to the changes in ionic currents and action potentials, an increased level of interstitial fibrosis and modifications in intercellular coupling have been reported in aged compared with adult tissue,99,100 and this may further enhance the susceptibility to AF with age. It is likely that Ca2+ handling is different between aged and adult atrial tissue, but this issue remains unexplored, especially in humans. AF is the most common cardiac arrhythmia seen in the clinic; it affects approximately 2 million people in the United States alone101 and is one of the main causes of embolic stroke.92 AF is characterized by rapid and irregular activation of the atria, at a rate of 5 to 10 Hz.92 Several studies have investigated the ionic mechanisms involved in the remodeling that occurs in the atria of patients with long-standing chronic AF, and have shown that electrophysiological remodeling contributes to the development of a substrate that facilitates the tendency for persistence of AF.102,103 Electrical remodeling induces changes in the biophysical properties of Na+, Ca2+, and K+ currents, which lead to shortening of the APD.104 Abnormal [Ca2+]i homeostasis also plays an important role in AF-induced electrical remodeling.105,106 These changes in E-C coupling have been reviewed comprehensively in a recent article,16 and the important underlying ionic mechanisms are discussed in the following section. Atrial cells from patients in chronic AF (cAF) display shorter APD compared with healthy patients in normal sinus rhythm (Figure 31-2, A).28,107–110 Furthermore, the normal human atrial APD90 shortens when paced at progressively faster frequencies, but in cAF, this shortening is severely attenuated.17 The peak amplitude of [Ca2+]i is reduced in atrial myocytes from cAF patients compared with those of healthy patients (Figure 31-2, B), although the SR Ca2+ content is unaltered.17 [Ca2+]i decays more slowly in cAF compared with sinus rhythm.17,112 Our recently published mathematical model of the human atrial cell provided novel insights into the ionic mechanisms underlying the altered APD/[Ca2+]i in cAF (see Figure 31-2, A, B; black: sinus rhythm, red: cAF); the salient points are discussed here. Bosch et al reported that the density of INa and the voltage dependence of steady-state activation were not altered in cAF in humans, whereas steady-state inactivation was shifted to the right by ≈10 mV,110 and no changes were detected in mRNA levels of the Na+ channel gene SCN5A.114 In contrast, data from Sossalla et al show that both expression of Nav1.5 and peak INa density are decreased (slightly) in the atrial myocardium of patients with cAF.115 Additionally, the late Na+ current component, INaL (see inset, Figure 31-2), was reported to be significantly increased in cAF patients.115 Sossalla et al proposed that this increase could be due to the increase in neuronal Na+ channel isoforms (Nav1.1 expression is increased),115 or it could be mediated by CaMKII, which is increased in AF111,116 and is known to regulate INaL,117 or to be caused by oxidative stress.118,119
Ionic Mechanisms of Atrial Action Potentials
Ionic Bases of Atrial Action Potentials in the Healthy Myocardium
Action Potential Characteristics
The Inward, Depolarizing Currents: (Na+, Ca2+)
The Outward, Repolarizing K+ Currents (K+)
[Ca2+]i, Electrogenic Pumps and Exchangers
Other Ion Channels
Ionic Bases of Regional Heterogeneity in Atrial Action Potentials
Ionic Bases of Atrial Action Potential Variations With Age
Action Potential and Ionic Remodeling in Chronic Atrial Fibrillation
Effects of AF on AP and [Ca2+]i
Ionic Remodeling in cAF
Sarcolemmal Ion Channels
INa
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree