Invasive Diagnostic Procedures
Umraan S. Ahmad
Matthew G. Blum
Invasive procedures are often indicated for the diagnosis and staging of chest diseases. Bronchoscopy, video-assisted thoracic surgery, pericardiocentesis, pericardial biopsy, transthoracic needle biopsy, and esophagoscopy are discussed elsewhere. Improvements in diagnostic imaging have improved the precision of invasive biopsies and is replacing the need for some invasive procedures. Nevertheless, thoracic surgeons still perform a large number of diagnostic procedures and should be familiar with their indications, contraindications, technical aspects, complications, and alternatives.
Mediastinoscopy
Mediastinoscopy remains one of the most common operations performed by most general thoracic surgeons. In 1954 Harken and associates first published a way to explore the superior mediastinum from a lateral approach after excision of the scalene fat pad.21 Carlens subsequently described the classic midline approach that led to the widespread use of this procedure.7 Its key features are the development of a pretracheal tunnel through a small single cervical incision and passage of a specifically designed lighted speculum, the mediastinoscope, that permits nodal sampling on each side of the trachea as well as access to the subcarinal nodes. Use of the Carlens’ procedure spread from Sweden to North America with the strong support of Pearson.46 Prior to computed tomographic (CT) and positron emission tomographic (PET) imaging, cervical mediastinoscopy was central to the staging of lung cancer prior to resection. Mediastinoscopy is now generally used more selectively as noninvasive imaging has improved. However, it remains an integral part of the evaluation and staging of patients with suspected lung cancer. It is also frequently used for the diagnosis of other causes of mediastinal lymphadenopathy or mass. With the introduction of videoscopic technology, a video mediastinoscope is used by some centers. The increasing use of esophageal and bronchial endoscopic ultrasound may decrease the need for mediastinoscopy, but at this time these should be considered complementary techniques.
Indications
The most common indication for mediastinoscopy is staging and diagnosis of lung cancer. Mediastinoscopy for diagnosing other mediastinal diseases is valuable but less frequent. Traditionally, mediastinoscopy was used for staging lung cancer because of the absence of reliable noninvasive methods for differentiating benign from malignant lymph nodes, the uniformly poor results of primary resection in patients with clinical N2 disease, and the frequency of N2 involvement. With improvement in the resolution of CT scanning and the addition of PET scanning, mediastinoscopy is being used more selectively. In addition to helping select patients for mediastinoscopy, PET and CT imaging can also be very helpful in directing mediastinal nodal biopsies. This is particularly true in cases of remediastinoscopy or for diagnostic biopsies in the setting of a previously treated lymphoma. For lung cancers, CT criteria for clinical node positivity rely on nodal size, with accepted short-axis diameter of 1 cm as the threshold (Table 18-1).12,59 The current guidelines of the American College of Chest Physicians, based on a meta-analysis of 35 studies with a pooled population of 5,111 patients, report a sensitivity of 51% and a specificity of 86% for CT scan alone.59 PET scanning, based on fluorodeoxyglucose (FDG) uptake, is also helpful in staging of the mediastinum or directing biopsies in the setting of lymphoma. Although the reported experience with PET scan assessment of the mediastinum varies, its negative predictive value is more than 90% in most lung cancer series.19,27,48,53 In contrast, the positive predictive value (PPV) of PET in these studies ranged from 43% to 63%. In patients who underwent both PET and mediastinoscopy, the negative predictive value (NPV) for PET was 98.4% and the PPV 49%.16 Meta-analysis of 44 studies with 2,865 patients reveals a sensitivity of 74% and a specificity of 85%.59 PET scanning has the additional benefit of identifying potential metastatic disease through whole-body scanning. When utilized in the presence of enlarged nodes on CT scan, PET scanning was found to have a median sensitivity of 100% and a median specificity of 78% on meta-analysis of 14 studies.15
It may be reasonable to bypass invasive mediastinal staging in some patients with a negative PET scan and without enlarged nodes on CT scan. This approach is based on the finding that false-negative PET results will occur in only about 5% of cases, whereas the false-negative rate of mediastinoscopy is approximately 10%. PET positivity, however, is often falsely positive. Therefore a positive scan cannot be relied on as definitive. Mediastinoscopy is warranted in the presence of uptake in the mediastinal nodes if presence of tumor in those nodes is the deciding factor for a course of therapy.
The differing findings in the literature have led some to recommend routine mediastinoscopy, whereas others use a
selective approach.28 In the latter group, common indications for mediastinoscopy in patients without radiographic mediastinal adenopathy include central tumor, known adenocarcinoma, the need for pneumonectomy, N1 nodal enlargement on CT, FDG uptake of nodal tissue by PET scan, superior sulcus cancers, and T4 tumors requiring caval or tracheal resections. A randomized study by the Canadian Lung Oncology Group comparing the rate of noncurative thoracotomy in routine versus selective mediastinoscopy found a trend favoring the selective strategy by both clinical and cost criteria.6 This study may be criticized because the routine mediastinoscopy patients did not have CT scans, whereas in current practice, all patients are studied by CT early in their evaluation.
selective approach.28 In the latter group, common indications for mediastinoscopy in patients without radiographic mediastinal adenopathy include central tumor, known adenocarcinoma, the need for pneumonectomy, N1 nodal enlargement on CT, FDG uptake of nodal tissue by PET scan, superior sulcus cancers, and T4 tumors requiring caval or tracheal resections. A randomized study by the Canadian Lung Oncology Group comparing the rate of noncurative thoracotomy in routine versus selective mediastinoscopy found a trend favoring the selective strategy by both clinical and cost criteria.6 This study may be criticized because the routine mediastinoscopy patients did not have CT scans, whereas in current practice, all patients are studied by CT early in their evaluation.
Table 18-1 Accuracy of CT, PET, and Mediastinoscopy for Staging the Mediastinum | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||
Contraindications and Cautions
Associated conditions that preclude safe mediastinoscopy include huge cervical goiter, extensive calcification or aneurysm of the innominate artery, and permanent tracheostomy after laryngectomy and radiation. Although not contraindications, several other factors may increase the difficulty of mediastinoscopy. A carotid bruit, especially on the right, should prompt extra care to minimize neck extension and innominate artery compression. Prior sternotomy or neck incision may make the midline harder to find but does not affect the deeper dissection because the pretracheal fascia is generally intact. Prior mediastinoscopy, in contrast, is associated with peritracheal fibrosis extending into the mediastinum. Repeat mediastinoscopy is nevertheless feasible in most cases and has been reported to be safe even in the setting of induction chemotherapy.36,40,61 Care must always be taken in separating the innominate artery from the trachea. Sharp dissection of adhesions under direct vision is safer than the blunt dissection approach used in first-time mediastinoscopy. If adhesions are very dense, the midline trachea–artery interface can sometimes be avoided by entering the pretracheal space from the left anterolateral aspect of the trachea. Mediastinoscopy can also be performed in the presence of superior vena caval obstruction.25 The most troublesome bleeding is from the superficial veins divided during dissection to the pretracheal fascia rather than the deeper veins, which are retracted laterally. Although generally feasible, complete multilevel nodal sampling during redo mediastinoscopy or mediastinoscopy in cases of caval obstruction may be difficult to perform safely. Fortunately all that is needed in most patients with caval obstruction is a tissue diagnosis. Repeat mediastinoscopy is usually done to determine the presence or absence of residual cancer after induction therapy. CT and PET imaging and knowledge of areas most likely to yield positive results may decrease the need for extensive node assessment. While mediastinoscopy is a useful diagnostic tool, it is not therapeutic. Hence aggressive mediastinoscopy in conditions that place the patient at substantial risk for catastrophic hemorrhage or other complication is not warranted.
Procedure
Cervical mediastinoscopy is generally performed as an outpatient procedure and can be combined with bronchoscopy, scalene biopsy, or anterior mediastinotomy as needed. Because of the possibility of significant bleeding, mediastinoscopy should probably be limited to hospital-based rather than free-standing facilities.
General anesthesia is induced. The patient is positioned supine with the occiput at the very top of the operating table. The neck is moderately extended by an interscapular roll in order to draw the trachea from the mediastinum into the neck. The back of the operating table is elevated slightly to decrease venous pressure. The prep includes the anterior chest and right chest for possible sternotomy and anterior thoracotomy respectively. The endotracheal tube exits the patient’s mouth on the anesthesiologist’s side. A pulse oximeter or radial arterial line is placed in the right extremity. This allows monitoring of the arterial waveform. A dampened waveform during manipulation of the mediastinoscope may indicate innominate compression, which can result in right carotid hypoperfusion. Optical magnification is not required but can be achieved with an attachable swing-away lens. Video mediastinoscopy is a recent option that allows both direct and monitor viewing. It aids in teaching by obviating the inevitable shifts that occur when the scope is handed back and forth and by assuring that all observers are viewing the same structures. It also provides substantial magnification, which may allow more precision with biopsies and improve identification of small structures such as the recurrent laryngeal nerve.
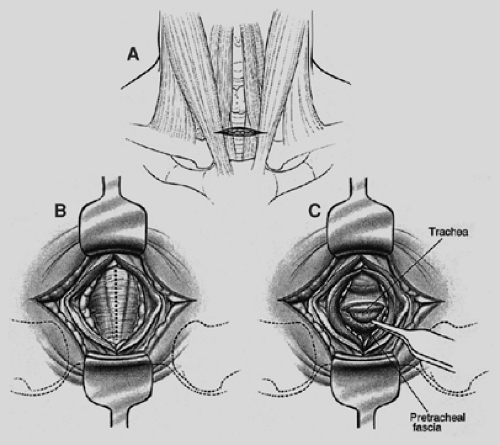
A 3-cm transverse skin incision is centered between the anterior borders of the sternocleidomastoid muscles 1 cm above the sternoclavicular junctions and carried through the platysma (Fig. 18-1). The avascular space between the strap muscles is opened vertically. Palpation of the trachea aids in locating the narrow midline space between the sternohyoid muscles. The deeper sternothyroid muscles are separated by a wide band of areolar tissue. The anterior wall of the trachea is now in view. If there is insufficient length of trachea caudal to the thyroid isthmus, this structure is retracted cranially. Rarely, the isthmus must be divided and oversewn or ligated. The thyroidima artery or a branch of the inferior thyroid vein must be ligated when these vessels cannot be retracted from the midline below the isthmus. It is essential to assess the area just above the sternal notch early in the dissection because cervical extension may draw the innominate artery up into the base of the neck, where it can be injured. The pretracheal fascia is incised and elevated. A tunnel is created in the pretracheal space using an index finger (Fig. 18-2). For purposes of palpation and later visualization,
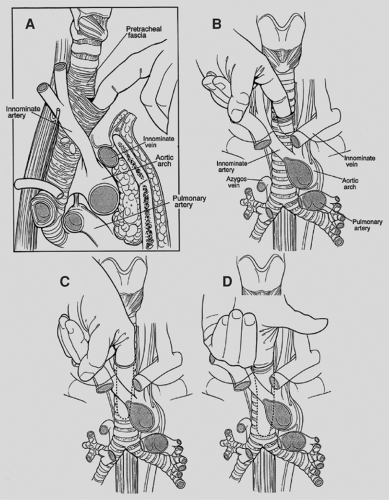
mediastinoscopy is a “tracheocentric” procedure; that is, anatomic orientation is always defined by the relationship to the trachea of the finger, the mediastinoscope, and the instruments. Tough fibrous bands between the trachea and fascia may be encountered proximally. Thereafter, gentle advancement and lateral sweeping clear the pretracheal and paratracheal spaces easily unless there is fibrosis or malignant invasion. The dorsal aspect of the finger senses the tracheal rings in stepladder fashion as it passes distally. Dissection is carried to the full length of the finger and usually reaches a point 1 or 2 cm proximal to the carina. In short patients, the carina may be reached and is identified as a midline posterior depression. Simultaneously, the anteriorly directed volar surface palpates the innominate artery and the aortic arch more distally. Pressures in the superior vena cava and pulmonary artery are too low for these landmarks to be appreciated. During digital dissection, the surgeon formulates a mental map of the area by noting the presence and location of normal structures as well as lymphadenopathy, mass, or invasion. Because the nodes are external to the pretracheal fascia, access requires penetration of the fascia. This is usually accomplished digitally at a point just beyond the innominate artery (Fig. 18-3). Enlarged nodes can then often be freed bluntly to a significant extent—a maneuver that facilitates subsequent endoscopic retrieval. Firm nodes in the upper right paratracheal area are particularly suitable because the finger can be hooked anteriorly around the innominate artery and blunt dissection performed against the chest wall. Alternatively, the pretracheal fascia can be penetrated later, using an instrument passed through the scope.
mediastinoscopy is a “tracheocentric” procedure; that is, anatomic orientation is always defined by the relationship to the trachea of the finger, the mediastinoscope, and the instruments. Tough fibrous bands between the trachea and fascia may be encountered proximally. Thereafter, gentle advancement and lateral sweeping clear the pretracheal and paratracheal spaces easily unless there is fibrosis or malignant invasion. The dorsal aspect of the finger senses the tracheal rings in stepladder fashion as it passes distally. Dissection is carried to the full length of the finger and usually reaches a point 1 or 2 cm proximal to the carina. In short patients, the carina may be reached and is identified as a midline posterior depression. Simultaneously, the anteriorly directed volar surface palpates the innominate artery and the aortic arch more distally. Pressures in the superior vena cava and pulmonary artery are too low for these landmarks to be appreciated. During digital dissection, the surgeon formulates a mental map of the area by noting the presence and location of normal structures as well as lymphadenopathy, mass, or invasion. Because the nodes are external to the pretracheal fascia, access requires penetration of the fascia. This is usually accomplished digitally at a point just beyond the innominate artery (Fig. 18-3). Enlarged nodes can then often be freed bluntly to a significant extent—a maneuver that facilitates subsequent endoscopic retrieval. Firm nodes in the upper right paratracheal area are particularly suitable because the finger can be hooked anteriorly around the innominate artery and blunt dissection performed against the chest wall. Alternatively, the pretracheal fascia can be penetrated later, using an instrument passed through the scope.
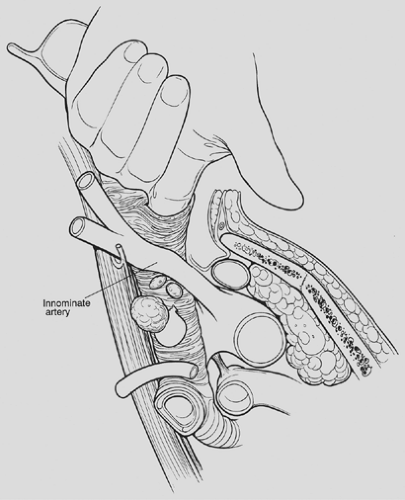 Figure 18-3. Oblique view of a finger piercing the pretracheal fascia to enter the node-containing space. Considerable blunt dissection of enlarged nodes can often be accomplished at this point. |
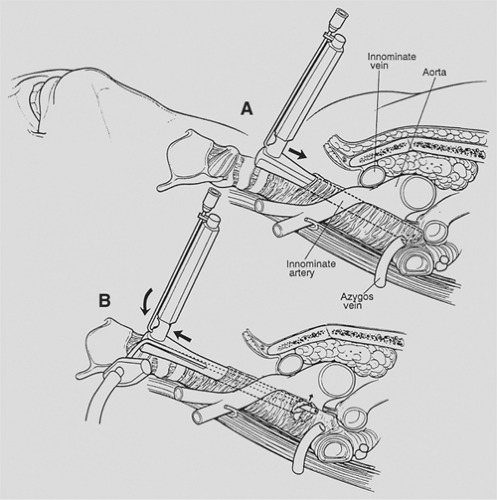
The mediastinoscope is now introduced. As in all endoscopic procedures, the instrument is advanced only if it passes easily and there is a visible tunnel. Passage is aided by blunt dissection with a metal or plastic suction–cautery device. It is important not to be confused by the position of the scope once it has been fully inserted. The pretracheal fascia and the slope of the trachea from anterior to posterior cause the tip of the scope to be focused directly on the airway or the subcarinal space. The mediastinoscope must be withdrawn slightly and angled anteriorly in order to enter the extrafascial space previously created digitally or to indent the fascia so that it can now be bluntly opened with the sucker (Fig. 18-4). The mediastinum is surveyed to confirm the findings of palpation by visualizing the pretracheal, paratracheal, and subcarinal nodes, azygos vein, pulmonary artery, carina and proximal mainstem bronchi, and sometimes the right upper lobe bronchial takeoff (Fig. 18-5). Lymph nodes are identified by color and consistency and by reference to the map generated during palpation. The blue-gray hue of venous structures may simulate the appearance of an anthracotic node. Pulsations may mislead both by their presence and absence because they may be transmitted to nonvascular structures and may be difficult to appreciate in the vena cava and azygos vein. If there is any doubt about the solid or vascular nature of the structure in question, aspiration is carried out using a 20-gauge spinal needle and small syringe. Saline in the syringe, as opposed to a dry system, facilitates identification of small amounts of blood. In the rare case of a completely solidified mediastinum, when anatomic definition is impossible and biopsy hazardous, aspiration cytology or core tissue histology may be sufficient. In most cases, however, it is possible to obtain substantial biopsy specimens. In order to prevent minor bleeding from obscuring the field, all target nodes are partially dissected before any biopsy specimens are taken. Node dissection through the scope is done bluntly, with the suction–cautery, endo dissectors, or biopsy forceps. Once sufficiently freed, the node is grasped with a cupped laryngeal biopsy forceps and traction is applied under direct vision. If the node cannot be extracted by gentle pulling and twisting, further dissection is carried out. It is occasionally possible and helpful to grasp the node and divide tethering attachments with a simultaneously introduced dissector or second biopsy forceps. If further digital dissection seems helpful, the scope is withdrawn and the finger reintroduced. Although removal of whole lymph nodes theoretically lessens the chance of tumor implantation and is esthetically pleasing, it is not always feasible owing to large size, friability, or adherence. In such cases, incisional biopsy specimens are taken with the laryngeal forceps. Particular care is taken in obtaining specimens near the tracheobronchial angles because of the proximity, on the right, of the azygos vein and apical branch of the anterior pulmonary artery and, on the left, of the recurrent laryngeal nerve. In dissecting around these structures, positive visualization and
identification will help decrease potential direct and traction injury.
identification will help decrease potential direct and traction injury.
The extent of biopsies depends on the clinical impression and operative findings. In lymphoma, sarcoidosis, or mediastinal mass, all that is needed is diagnostic tissue. For staging lung cancer, on the other hand, it is necessary to obtain specimens from several locations. Biopsies are taken from the high and low right and left paratracheal regions and from the subcarinal space (levels 2, 4, 7). If there is unequivocal extranodal tumor at high or contralateral stations, additional levels need not be sampled because staging will not be altered. In general, however, the time taken for frozen section confirmation is greater than the time and risk involved in taking more specimens. Routine biopsy of nodes contralateral to the primary tumor is essential in order not to understage N3 cases. Labeling all specimens by numerical station is less subject to error than is using their anatomic designations. In addition to biopsy of nodal stations, mediastinoscopy can be used to distinguish tracheal invasion versus abutment of central tumors.
Bleeding is usually minor and requires no treatment. To assess hemostasis, the scope is withdrawn slowly. Most bleeding can be controlled by packing with vaginal packing gauze for a few minutes. Oxidized cellulose can be packed into the subcarinal and paratracheal spaces and left in place. If there is a visible bleeding vessel, it can be clipped. Clipping is most useful for bronchial vessels in the subcarinal space. If cautery is used, the power should be kept low, and it should be applied directly to the bleeding structure in order to avoid arcing and thermal damage.
If there is concern about pleural or pulmonary puncture, the tunnel is filled with saline. Rapid disappearance of the liquid may indicate a pleural rent. Bubbling suggests a parenchymal communication. If the leak is significant, a chest tube is placed. For closure, the strap muscles are reapproximated in the midline with one or two absorbable sutures. Ridge formation is lessened if the platysma is not closed separately. A subcuticular closure is used for the skin. Drainage is not necessary.
Variations
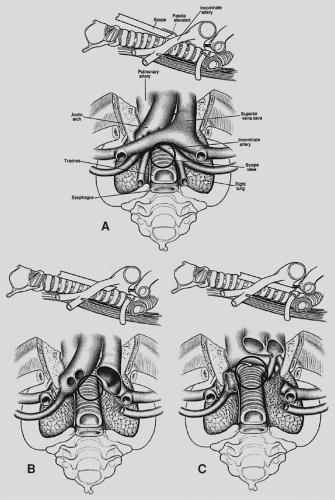
In some cases, standard cervical mediastinoscopy offers inadequate access for staging by one procedure. In such instances, cervical mediastinoscopy can be combined with anterior mediastinoscopy or thoracoscopy. Extended cervical mediastinoscopy has been described in which aortopulmonary (level 5) and anterior mediastinal (level 6) nodes are accessible through a cervical incision (Fig. 18-6).14,33,41 Following clearing of the infraisthmic trachea or completion of standard mediastinoscopy, the space anterior to the aortic arch is opened by digitally piercing the fascia between the innominate and left common carotid arteries. The innominate vein, separated from the operative area in standard mediastinoscopy by the arch arteries, now lies directly anterior to the tunnel. Following palpation, the mediastinoscope is passed anterior to the aorta, and appropriate biopsy specimens are taken.
Mediastinopleuroscopy
In mediastinopleuroscopy the pleural space is intentionally entered (Fig. 18-7). On the right, the pleura is opened posterior to the innominate artery, whereas on the left, entry is gained between the left common carotid and left subclavian arteries. In addition to pleural biopsy and fluid sampling, small upper-lobe lung biopsies can be obtained.11 However, the risk of seeding a clean mediastinum by transpleural biopsy of an upper-lobe cancer or infection must be considered.
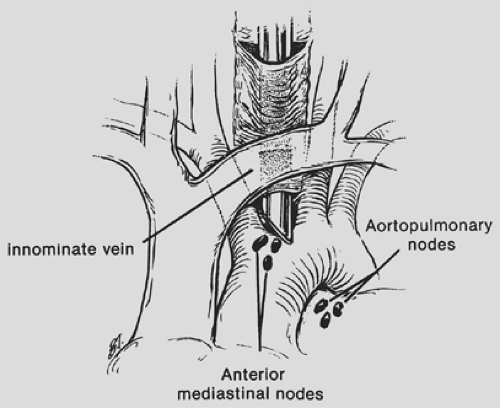 Figure 18-6. Extended mediastinoscopy.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|