Introduction to Statistics in Clinical Research Interventional Cardiology
Introduction to Statistics in Clinical Research Interventional Cardiology
Robert A. Harrington MD
Karen S. Pieper MS
Clinical cardiologists, including interventional specialists, need to understand the quantitative issues in clinical research so that they are capable of choosing therapies and technologies that have proven benefit for their patients and avoiding therapies and technologies that are either harmful or unlikely to provide benefit to their patients. In this chapter, we discuss the basic concepts of evidence-based medicine, address basic issues of designing clinical studies, and discuss common analytical techniques. All of these topics should be familiar to clinicians, whether or not they are engaged in clinical investigation. Interventional cardiology is a continuous learning process; an ability to read and interpret the medical literature with facility is an important part of one’s professional life.
Clinical research provides the evidence upon which the practice of medicine is best and most reliably based. The results of clinical studies are typically subjected to peer review before being published in leading medical journals. However, when making decisions about care to offer to his or her patients, the individual clinician needs to have a level of comfort with and understanding of basic quantitative methods so that he or she can arrive at the most appropriate medical decisions.
EVIDENCE-BASED MEDICINE
Evidence-based medicine has been defined as combining quantitative evidence about medical practice with expert judgment in an effort to ensure that medical care is provided with reproducible high quality (
1). In addition to understanding the concept of evidence-based medicine, those preparing for the Interventional Cardiology Board examinations must be familiar with the various American College of Cardiology/American Heart Association (ACC/AHA) guidelines for clinical practice.
The ACC/AHA Guidelines Committees have issued a series of evidence-based practice recommendations that cover a variety of diseases and cardiac procedures, including percutaneous coronary intervention (PCI) and coronary bypass surgery (
2,
3). These Guidelines are constructed using a defined, objective methodology that begins with the accumulation and weighing of evidence. All recommendations are accompanied by an assigned evidence grade (A, B, or C) (
Table 12-1). Grade A means that the data have been derived from many trials or at least a single large, randomized clinical trial. Grade B indicates that the data have been derived from smaller randomized trials or nonrandomized observational studies. The lowest weight of evidence is a grade C, which denotes expert consensus.
After weighing the evidence, guideline writers, including clinical experts, statisticians, and policy makers, assign classes of recommendations (I, II, or III) (
Table 12-1). Class II recommendations are further subdivided into IIa and IIb. For the purposes of Board preparation, the candidate must be particularly familiar with Class I and III recommendations. Class I recommendations are those in which the intervention is felt to be useful and effective; Class III denotes interventions that are not deemed useful or effective, and may in fact be harmful. The Class II recommendations indicate situations where the evidence has been more controversial. A Class IIa recommendation is given when the evidence conflicts or opinions differ, but overall the evidence leans toward benefit; a Class IIb recommendation indicates that the evidence conflicts or opinions differ, but the weight of the evidence leans against benefit. Despite a wealth of information from clinical trials supporting many of the major decisions in cardiovascular medicine, including interventional cardiology, the majority of recommendations in the ACC/AHA guidelines are Class II recommendations, suggesting that a limited amount of evidence exists even for interventions considered routine in clinical practice.
TYPES OF STUDY DESIGNS
There are two main types of study design:
observational studies and experimental studies (
4). In observational studies, patients or groups of patients are observed over a period of time, and their characteristics are recorded. In an experimental design, an intervention such as a drug, procedure, or technology is introduced into the population, and the effect on the study subjects is observed.
Types of observational studies vary. We will consider a few with which the reader should be familiar. These include case-control studies and cohort studies. Regarding experimental studies, we will mainly consider randomized controlled trials.
Case-Control Studies
A case-control study is typically performed on previously collected, retrospective data. In these studies, there are five steps to consider.
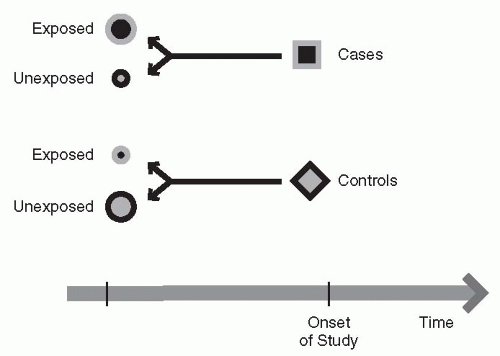
First, one begins with either the presence or absence of an outcome of interest. Second, one defines a group of cases that have this measure of interest—typically, this is a disease or an outcome event. Third, a control group is identified that does not have the disease or the measure of interest, but may be matched on common characteristics such as age or gender. Fourth, the investigator looks at the history of cases to detect possible causes or risk factors that were not controlled for in the matching process. Step five is an attempt to answer the question of what happened or what is the difference between the cases and the controls that might explain the outcome of interest. A case-control study might be useful, for example, to consider whether the use of a medication is related to the development of a disease. When considering a treatment, the cases and controls should be carefully matched on important risk factors in order to be as certain as possible that only the treatment effect varies between the two groups.
Figure 12-1 and
Table 12-2 show examples.
Case-control studies have certain advantages and disadvantages. This may be the best design for studying diseases or conditions that develop over a long time or are extremely rare. They may be very useful for investigating a preliminary hypothesis, because they are typically a very rapid way of performing a study, provided the data on the population have already been collected. The major disadvantage is that the case-control study depends on existing records, which may have been collected for other reasons. This particular study design is subject to a fair degree of bias or error, because the data are typically collected in advance of the question being asked, so one is limited by the existing data. For example, factors associated with the outcome may not be equally distributed between the two treatments. If these factors are not available to be examined, one cannot test whether the differences in the factors, rather than the treatment being studied, are responsible for any statistically significant treatment results. In addition, patient care may have changed since the data were collected, making the results no longer applicable. Choosing an appropriate control group (including one that is matched for certain characteristics) is critical, but may prove to be quite difficult.
Cohort Studies
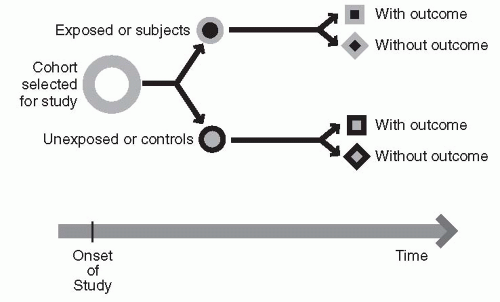
In a cohort study, information is collected on a group of subjects who have something in common and who remain part of that group for an extended period of observation or follow-up. Typically, in this type of design, one begins with the identification of an exposure to some event that is felt to be relevant to the development of some outcome in the future. One then identifies two groups of subjects, the exposed group and the nonexposed group. One then looks forward in time from the exposure to determine the effect of the defining characteristics or exposure on the outcome of interest. This design attempts to answer the prospective question: what will happen?
An example of a cohort study design is seen in
Figure 12-2 and
Table 12-3. A cohort study is a good design when one is interested in studying the particular causes of a condition, the course of a particular disease, or the impact of risk factors over time. The Framingham Study, which has provided so much critical information on the understanding of the association between cardiac risk factors and cardiac outcome, is an example of a cohort study (
5). One of the major disadvantages of a cohort design is that studies such as the Framingham study may take a long time to conduct. Because of this, they tend to be resource-intensive. It is also a difficult methodology when one is interested in causation: one may define association, but because there is no intervention being introduced into the population, it is difficult to prove causation. It may also be a difficult design when a disease is rare in the population, because the requisite large sample size may be prohibitive.
EXPERIMENTAL STUDIES
There are two types of experimental studies: the controlled and the uncontrolled study. In a controlled study, an experimental drug, procedure, or technology is typically compared with at least one other drug, procedure, or technology. This might include a comparison with placebo. In an uncontrolled study, an investigator will
describe the experience with the experimental drug or procedure, but not compare it directly with another treatment. This type of experiment has less validity and is less likely to allow one to conclude that there are differences between the treatments.
Controlled clinical trials may be further grouped into two types: randomized and nonrandomized experiments. In each of these, the trial is conducted with concurrent controls. There are typically two groups: the experimental group, who receive the experimental drug or procedure, and the control group, who receive placebo or a standard drug or procedure. Randomized clinical trials provide the strongest evidence for reaching a conclusion of causation, whereas in the nonrandomized trial, when the assignment to a treatment group is not random, there may be biases introduced that render conclusions questionable.
Get Clinical Tree app for offline access