Intravascular Ultrasound
Vitalie Crudu MD
John McB. Hodgson MD, FSCAI, FACC
Intravascular ultrasound (IVUS) allows direct visualization of coronary and vascular anatomy during diagnostic and interventional catheterization. Unlike angiography, which depicts a silhouette of the coronary lumen, IVUS displays a tomographic, cross-sectional perspective. This facilitates direct measurements of the lumen dimensions, including the minimum and maximum diameter and cross-sectional area (1). By employing a timed pullback, length measures may be obtained. Ultrasound-derived measurements are more accurate than are angiographic dimensions (2). In addition to luminal measurements, the ability of coronary ultrasound to image the soft tissues within the arterial wall enables characterization of atheroma size, plaque distribution, and lesion composition (3). Accordingly, ultrasound can detect the presence or absence of structural abnormalities of the vessel wall after mechanical interventions, including dissections, tissue flaps, intramural hematomas, perforations, and irregular surface features. Since intracoronary ultrasound was first performed in 1988, it has been instrumental to our understanding of coronary anatomy and pathophysiology and has allowed detailed evaluation of interventional procedures (4).
IVUS DEVICES
IVUS equipment requires two components: a catheter incorporating a miniaturized transducer and a console containing the necessary electronics to reconstruct an ultrasound image. Catheters typically range in size from 2.9 to 3.5 Fr (0.96 to 1.17 mm). Two technical approaches to transducer design have emerged: mechanically rotated imaging devices and a multielement electronic array device. The mechanically rotated design requires an imaging sheath; the electronic design is inserted directly into the artery. Most systems use a monorail design to facilitate rapid catheter exchange. In clinical practice, both devices provide accurate information for guiding patient care.
ARTIFACTS AND LIMITATIONS
Mechanical transducers may exhibit variations in rotational speed arising from mechanical drag on the catheter driveshaft, creating nonuniform rotational distortion (NURD) and producing visible distortion. NURD is most evident when the driveshaft is bent into a small radius of curvature by a tortuous vessel and is recognized as circumferential “stretching” of a portion of the image with “compression” of the contralateral vessel wall. An additional artifact, transducer ring-down, appears in virtually all medical ultrasound devices. This artifact arises from acoustic oscillations in the piezoelectric transducer material, resulting in high-amplitude signals that obscure near-field imaging. In mechanical systems, this artifact may be merged with the imaging sheath artifact. In electronic array catheters, this artifact may be largely removed by mask subtraction. All intravascular imaging systems are vulnerable to geometric distortion produced by oblique imaging. Thus, when the ultrasound beam interrogates a plane not orthogonal to the vessel walls, an artery with a circular lumen appears elliptical in shape. Some transducer designs position the guide wire external to the transducer, thereby introducing an obligatory “wire artifact.” In general, higher-frequency transducers have a lower penetration depth; in practice, this is not usually an issue for coronary imaging, but may become evident if peripheral arterial imaging is attempted. Alternative catheters with a lower ultrasound frequency are used for large-vessel peripheral imaging and for intracardiac examination.
SAFETY OF CORONARY ULTRASOUND
Although IVUS requires intracoronary instrumentation, initial studies conducted during diagnostic catheterization demonstrated few serious untoward effects. The technique has been shown to be safe in the long term and no acceleration of atherosclerosis because of catheter-induced endothelial damage has been demonstrated (5). The imaging transducer can transiently occlude the coronary when advanced into a tight stenosis or a small distal vessel, but patients generally do not experience chest pain if the catheter is promptly withdrawn. Preinstrumentation nitroglycerine is advised to prevent spasm. Adequate anticoagulation is required prior to catheter insertion. In interventional practice, operators have safely used coronary ultrasound after most types of procedures, including balloon angioplasty and stent deployment. Despite the relative safety of coronary ultrasound, any intracoronary instrumentation carries the potential risk of intimal injury or acute vessel dissection.
QUANTITATIVE LUMINAL MEASUREMENTS
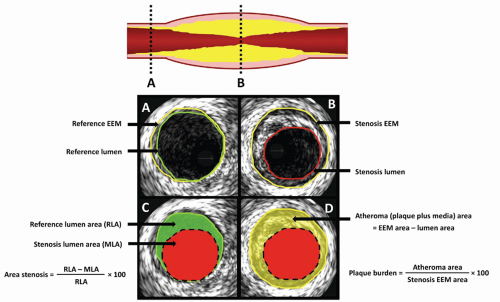
Diagnostic and interventional practitioners routinely use luminal measurements to evaluate the severity of stenoses, determine the size of the “normal” reference segment, and assess gain in lumen size achieved by revascularization (Fig. 8-1). Comparisons of vessel dimensions by angiography and IVUS generally reveal a limited correlation, particularly for vessels with an eccentric luminal shape, presumably owing to the inability of angiography to accurately portray the complex, irregular cross-sectional profiles of atherosclerotic vessels. In general, angiography overestimates lumen dimensions compared with IVUS, even after symmetric stent implantation. By performing a timed or calibrated pullback through the vessel, a third dimension of information (length) can be collected, allowing the calculation of the lumen, vessel, and plaque volume, as well as plaque composition. This information has been instrumental in evaluating the mechanism of different interventional techniques, the pathology of restenosis, and the effect of drug therapy aimed at treating atherosclerosis.
ANGIOGRAPHICALLY UNRECOGNIZED DISEASE
IVUS commonly detects atherosclerotic abnormalities at angiographically normal coronary sites. The long-term implications of these findings remain uncertain. In the Prospective Natural-History Study of Coronary Atherosclerosis Trial (PROSPECT), Stone et al. have demonstrated that, after an acute coronary syndrome, most major adverse cardiovascular events involving nonculprit lesions at follow-up were caused by angiographically mild lesions (32.3% ± 20.6% stenosis at baseline), of which 30.2% were angiographically inconspicuous (<30% stenosis) (3). Accordingly, the presence of angiographically occult coronary disease may have important prognostic significance. Studies are currently underway to determine the predictive value of IVUS in determining the prognosis in patients with coronary disease. In PROSPECT, a plaque burden of 70% or greater, a minimal lumen area (MLA) of 4.0 mm2 or less (in the proximal epicardial vessels), and thin-cap fibroatheromas by radiofrequency IVUS were more likely to be associated with recurrent events. Several studies have shown that plaque burden or lumen size in the left main coronary may be an indicator of the behavior of the coronary vasculature and that left main disease seen on IVUS predicts adverse cardiac events (6).
Using volumetric intracoronary ultrasound, minor changes in plaque and lumen volume can be reliably detected. By comparing baseline and repeat studies, the effects of drug therapy on atheroma can be assessed. Owing to the precise measures, such methodology allows pharmaceutical studies to be completed using far fewer patients than necessary if only clinical endpoints are collected (7).
Detection of the impact of calcium on the response of lesion to intervention is one of the most important contributions of IVUS imaging. Calcium is frequent in target lesions (75%), but poorly detected by angiography (sensitivity only 40%) (8).
LESIONS OF UNCERTAIN SEVERITY
Despite thorough radiographic examination with multiple projections, angiographers commonly encounter lesions that elude accurate characterization. Coronary atherosclerosis can be associated with vessel remodeling and dilatation; thus the angiographic appearance of the vessel may be normal despite significant accumulation of plaque. Lesions of uncertain severity often include ostial lesions and moderate stenoses (angiographic severity ranging from 40% to 70% diameter stenosis) in patients whose symptomatic status is difficult to evaluate. For these ambiguous lesions, ultrasound provides tomographic measurements, enabling quantification of the stenosis independent of the radiographic projection. Bifurcation lesions are particularly difficult to assess by angiography because overlapping side branches often obscure the lesion. Based on earlier studies, an MLA ≥4.0 mm2 can be safely used as a cutoff for
identifying nonischemic lesions for which PCI can be deferred (9). However, using a cutoff MLA to predict which lesions will result in stress-induced ischemia (or a low fractional flow reserve [FFR]) should be avoided, because other lesion characteristics, such as length, area stenosis, plaque burden, reference vessel size, and location, are important contributors to the hemodynamic significance of a lesion (10). In most situations, physiologic measures, such as FFR, are better suited and should be considered the gold standard for assessing the significance of intermediate lesions, or decisions regarding revascularization.
identifying nonischemic lesions for which PCI can be deferred (9). However, using a cutoff MLA to predict which lesions will result in stress-induced ischemia (or a low fractional flow reserve [FFR]) should be avoided, because other lesion characteristics, such as length, area stenosis, plaque burden, reference vessel size, and location, are important contributors to the hemodynamic significance of a lesion (10). In most situations, physiologic measures, such as FFR, are better suited and should be considered the gold standard for assessing the significance of intermediate lesions, or decisions regarding revascularization.
LEFT MAIN LESIONS
As the severity of left main disease is of critical importance for properly determining patient treatment, intravascular imaging of this vessel deserves special mention. The normal diameter of the left main has been characterized by IVUS. Using the definition of the mean minus two standard deviations, the lower limit of normal, nondiseased left main luminal area is found to be 7.5 mm2 (11). With respect to functionally significant lesions, comparison with FFR has revealed that the best cutoff points for predicting an FFR <0.75 is an IVUS left main diameter of <2.8 mm or area <5.9 mm2 (12). Further, in the large, nonrandomized, 354-patient LITRO study, a left main MLA cutoff of 6 mm2 was prospectively validated to be safe for determining which patients require revascularization. In this study, the 179 patients with a left main MLA above 6 mm2 for whom left main revascularization was deferred had similar outcomes to the revascularized group. Only eight patients required subsequent left main revascularization after 2 years of follow-up (13). The negative impact of left main disease discovered by IVUS on patient prognosis is well known. The 2011 ACC/AHA/SCAI guidelines for percutaneous coronary interventions (PCI) assign a class IIa recommendation for performing IVUS to assess angiographically indeterminate left main lesions (14). Given the above-mentioned limitations of MLA in assessing the hemodynamic significance of a stenosis, FFR is also appropriate for assessing intermediate left main lesions.
IVUS guidance during the intervention may affect long-term outcomes after left main stenting. In an analysis of the MAINCOMPARE registry using propensity-score matching, IVUS guidance was associated with reduced 3-year mortality (in the subset of patients receiving drug eluting stents [DES]) compared with angiographic guidance (4.7% vs. 16%, p = 0.048) (15). Prospective randomized data regarding IVUS guidance for left main stenting are currently lacking, although current consensus is that IVUS should be used during all unprotected left main interventions.
CORONARY STENT DEPLOYMENT
IVUS has significantly influenced our understanding of the mechanism underlying stent deployment and is now widely used in guiding clinical procedures. A small observational study documented that angiographically guided stent deployment using first-generation stents and low-pressure techniques resulted in an average residual stenosis of 51% (comparison of minimum stent diameter with reference segment diameter measured by IVUS) (16). Subsequently, this same center used IVUS criteria and highpressure balloon postdilation to optimize stent expansion with reduction in residual stenosis to —7 ± 16%. This study reported a subacute thrombosis rate of only 0.3% with antiplatelet agents alone, and forever altered the technique of stent implantation, allowing elimination of Coumadin from the poststent regimen (17).
The mechanism of deployment, and subsequently criteria for optimal ultrasound-guided bare-metal stent (BMS) deployment, have been extensively explored (18). To minimize restenosis, most authorities recommend that operators attempt to achieve a minimum stent lumen cross-sectional area of over 8 mm2 when vessel size allows (19). High pressure (>16 atm) and upsized balloons (by 0.25-0.5 mm) are sometimes necessary to achieve these results.
Calcium in the lesion is an important limitation to appropriate stent expansion, even after high-pressure balloon dilation (20). Pretreatment with rotational atherectomy improves stent results in these calcified lesions.
Most trials on angiographic versus IVUS guidance for optimizing BMS deployment have suggested a benefit for IVUS guidance. In a recent meta-analysis of seven randomized trials (21) comparing IVUS versus angiographic guidance for BMS PCI involving 2,193 patients, IVUS guidance was associated with a larger postprocedure angiographic MLA (mean difference of 0.12 mm, 95% CI: 0.06-0.18, p < 0.0001), lower rate of 6-month angiographic restenosis (22% vs. 29%, OR: 0.64, 95% CI: 0.42-0.96, p = 0.02), significant reduction in the revascularization rate (13% vs. 18%, OR: 0.66, 95% CI: 0.48-0.91, p = 0.004), and overall reduction in major adverse cardiac events [MACE] (19% vs. 23%, OR: 0.69, 95% CI: 0.49-0.97, p = 0.03). There was no difference in the rates of myocardial infarction (p = 0.51) or mortality (p = 0.18) (Figs. 8-2, 8-3, 8-4 and 8-5).
It is generally accepted that angiographic appearance after intervention can be deceiving, and IVUS can be very useful in determining suboptimal stent expansion and quantifying the additional gain achieved by higher pressure or larger diameter repeat balloon dilation. Additional studies have documented benefit for ultrasound guidance in small vessels (22) and long lesions (23), as well as high-risk bifurcation and left main lesions (24, 25). The finding that many “small” vessels are actually not small but severely diseased has justified oversized stenting in these positively remodeled vessels. Definitive evidence to support routine IVUS guidance of all stent procedures is lacking, although positive studies in varied subgroups suggest that IVUS can be of benefit in many stent procedures.
Data from core laboratories with careful clinical follow-up have allowed investigation of particular IVUS findings. Minor stent malposition (SM) (26) or small edge tears (27) have not adversely affected patient outcome after stenting, and may be left untreated.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree