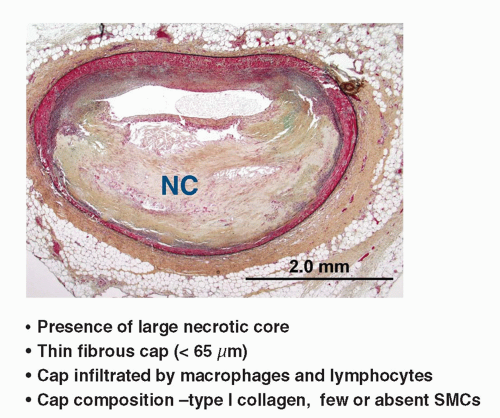
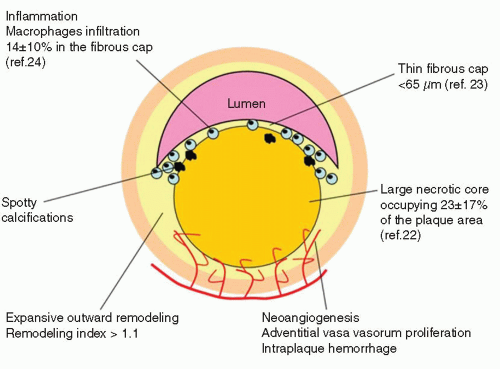
 FIGURE 9-2 Typical morphologic traits associated with rupture prone plaques. (From: Vancraeynest D, et al. J Am Coll Cardiol. 2011;57:1961-1979, with permission.) |
Architecture: Plaque volume, length, remodeling, eccentricity, impact on lumen area, fibrous cap thickness, evidence of disruption;
Physiology: Impact on coronary flow reserve;
TABLE 9-1 Histologic Features of Suspected Vulnerable Plaquesa
Type
Features
nflamed thin-cap fibroatheroma, most frequent type and present in 60% to 70% of ACS cases
Necrotic core (lipid core) Thin cap <65µm nflammation
Erosion site, present in 20% to 30% of events, more frequent in women and younger patients with ACS
Increased vasa vasorum
Expansive remodelling
Increased plaque burden
Intraplaque hemorrhage
Calcified nodule, present in <3% of patients with ACS
Spotty calcification
Luminal narrowing
Plaque with a mural thrombus not producing a significant stenosis
Increased proteoglycans
Extensive calcification protruding into lumen
Considered to be a site of subsequent thrombosis because many plaque-causing events show repeated episodes of disruption and thrombosis
ACS, acute coronary syndrome; Ml, myocardial infarction; PCI, percutaneous coronary intervention; TCFA, thin cap fibroatheroma
aaFrom: Muller JE et al. J Am Coll Cardiol Imaging. 2010;3:881-891
Composition: Lipid-necrotic core, fibrous, hemorrhage, calcium, etc.;
Pathobiology: Presence of inflammation, neovascularization, fibrous cap metabolism, apoptosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree