Investigator
No. of CEAs
% Revised
No. of ICA occlusions
Restenosis at 2–3 year
Baker et al. [3]
316
3
3 (1%)
3%
Papanicolaou et al. [4]
86
11
2 (2%)
0
Panneton et al. [5]
155
9
0
2%
Bandyk et al. [2]
390
8
1 (0.3%)
2%
Ascher et al. [6]
650
3
0
2%
Schanzer et al. [10]
407
8
0
2%
While the goal of intraoperative assessment is the detection of repair site lesions, a secondary benefit is recognition of abnormal repair site hemodynamics with the potential to cause recurrent stenosis. Outcome analysis of carotid endarterectomy sites with altered blood flow characteristics such as moderate elevation (150–200 cm/s) in peak systolic velocity (PSV) or residual plague producing <30% lumen stenosis has been shown to reduce the functional patency by development of intimal hyperplasia. The natural history of recurrent stenosis is more benign than an atherosclerotic plaque, but progressive intimal hyperplasia can lead to internal carotid occlusion. Most vascular surgeons recommend repair of asymptomatic recurrent stenosis with duplex findings of a >80% diameter reduction (DR) stenosis [2–4]. Treatment is typically by stent-angioplasty. Similarly, the development of in-stent stenosis is most commonly the result of intimal hyperplasia and, if progressive to a high-grade stenosis, can cause stent occlusion. Following both endarterectomy and stent-angioplasty, the likelihood of recurrent stenosis has been shown to be associated with the presence of residual stenosis [1, 2, 4].
Carotid Endarterectomy Duplex Scan Protocol and Test Interpretation
Intraoperative duplex scanning of carotid repairs is performed after restoration of ICA blood flow using a “hockey stick” linear array 10–15-MHz ultrasound transducer enclosed in a sterile plastic sheath (Fig. 16.1). Acoustic coupling for vessel imaging is achieved by ultrasound gel in the sheath and saline in the incision. Imaging is accomplished by placing the transducer over the artery and then slowly moving it along the repair beginning in the common carotid artery and proceeding distally to the ICA. If a prosthetic (bovine, polyester) patch was used for vessel closure, lumen imaging is still possible, but use of a polytetrafluoroethylene (PTFE) patch hampers imaging due to air trapped in the PTFE material. Vessel imaging and velocity spectra recordings can be carried by orienting the transducer foot pad along the nonpatched vessel circumference. With the assistance of a vascular technologist to optimize instrument setting for imaging and pulsed Doppler spectral analysis, the exam time is less than 10 min including archiving images for the patient medical record.
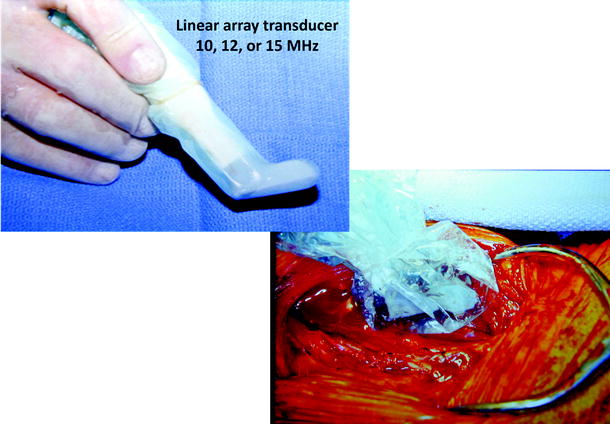

Fig. 16.1
A “hockey stick” linear array (10–15-MHz) transducer suitable for intraoperative carotid repair site imaging. The small footprint transducer is enclosed in a sterile plastic sheath for scanning within the neck incision directly on the carotid endarterectomy site
Duplex scanning should begin at the proximal common carotid artery (CCA) to verify normal proximal endarterectomy end point. The site of proximal clamp occlusion should be imaged since traumatic wall dissection could have occurred. Scanning then proceeds from proximal to distal to confirm a widely patent lumen and normal velocity spectra (Fig. 16.2). Special attention should be paid to endarterectomy end points where residual plaque >2 mm in thickness is abnormal and should be repaired. The normal endarterectomy site should be free of lumen defects, no suture stricture, and PSV should be <150 cm/s (Figs. 16.3 and 16.4). The criteria for an “abnormal” duplex scan depend on the site (CCA, ICA) imaged and the severity of the anatomic defect and associated hemodynamic changes indicating stenosis. Using transverse imaging, the diameter of the proximal ICA (bulb segment) should demonstrate homogenous color flow, and the diameter can be measured which should be <1 cm as larger diameter patched segments are prone to aneurysmal dilation and mural thrombus formation (Fig. 16.5). The external carotid artery (ECA) is imaged to verify patency and presence of distal plaque dissection since the endarterectomy of this vessel is accomplished using an eversion technique for plaque removal. Most surgeons will reexplore the ECA if occlusion or a focal high-grade stenosis is identified. The ICA should be scanned as far distal as possible especially if a shunt was inserted. When an anatomic defect (plaque edge, ICA suture narrowing, artery kink) is imaged, walking the sample volume through the region allows assessment of changes in PSV. Decision for repair is based on the altered hemodynamics produced by the imaged abnormality with lesions producing focal elevations of PSV > 150 cm/s considered for repair (Fig. 16.6). Spasm of the ICA is identified by a narrow lumen on color or power Doppler and moderate elevations of PSV in the range of 150–200 cm/s. The finding of lumen thrombus and PSV > 300 cm/s usually indicates platelet aggregation has developed, and reexploration is mandatory. Many vascular surgeons will perform angiography when the duplex imaging is abnormal to confirm an anatomic defect prior to proceeding with endarterectomy site reexploration.
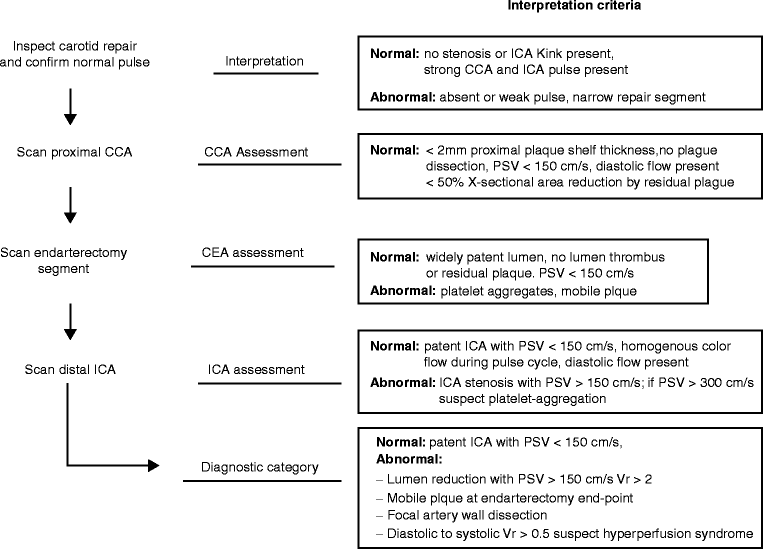
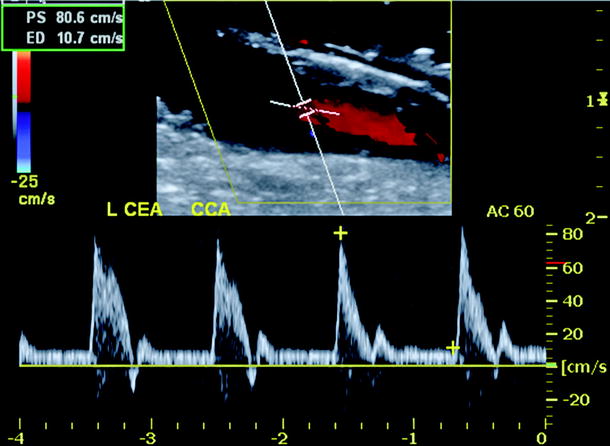
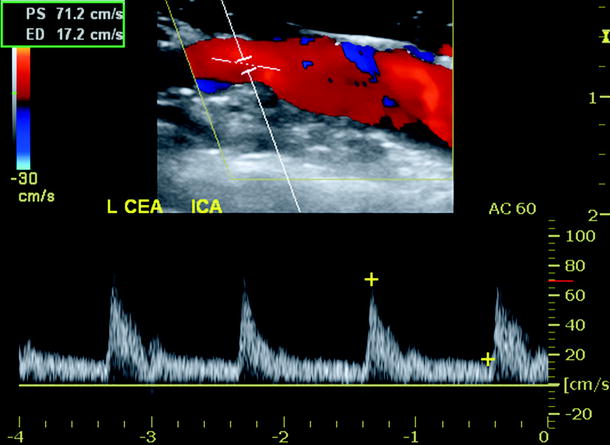
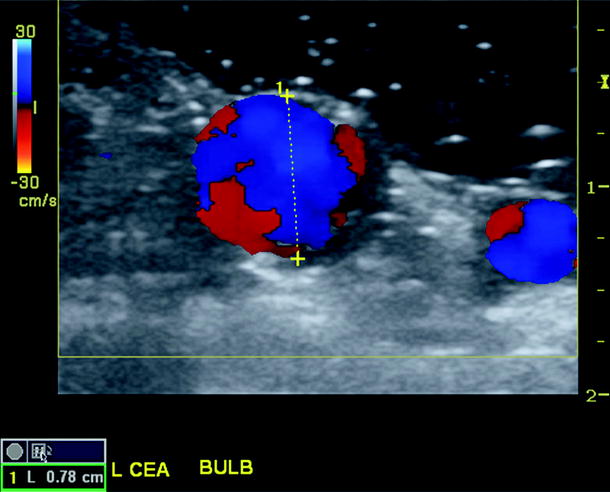
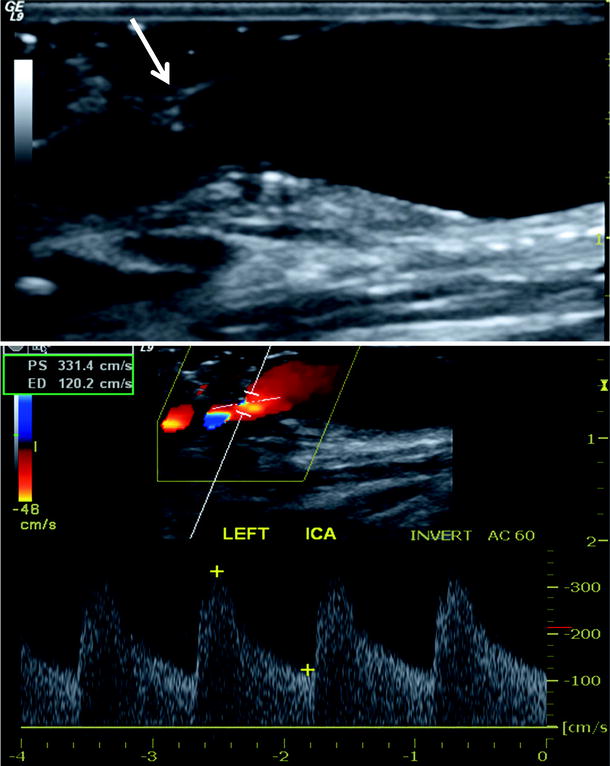

Fig. 16.2
The duplex ultrasound imaging protocol and interpretation criteria after carotid endarterectomy

Fig. 16.3
Duplex ultrasound image of common carotid artery endarterectomy end point showing mild residual plaque but normal velocity spectra (minimal spectral broadening, peak systolic velocity <150 cm/s)

Fig. 16.4
Duplex ultrasound image of vein patch endarterectomy site with normal color Doppler imaging and velocity spectra (mild spectral broadening, peak systolic velocity <150 cm/s)

Fig. 16.5
Transverse duplex scan image of reconstructed carotid bulb showing homogenous color flow, widely patent lumen, and a measured diameter of 0.78 cm

Fig. 16.6
Abnormal duplex scan of carotid endarterectomy site after polyester patch angioplasty. Residual plaque (arrow) is present on anterior wall which narrows the ICA lumen producing elevated peak systolic velocity >300 cm/s with severe spectral broadening. Meets criteria for immediate reexploration
On occasion, increased PSV ≥ 125–150 cm/s with minimal spectral broadening and normal artery imaging can be the result of vasospasm or compensatory collateral flow due to contralateral ICA occlusion. The presence of high diastolic flow in the ICA, i.e., more than 50% of the PSV, may indicate hyperperfusion syndrome with loss of normal intracranial arterial autoregulation. Appropriate therapy for this condition may include meticulous blood pressure control, steroids, and antiseizure drug administration.
The prevalence of endarterectomy site repair based on duplex testing is approximately 5% for CCA and ICA defects (Table 16.1), and additional 3–5% if correction of ECA stenosis/occlusion is included. If the endarterectomy site has normal duplex imaging and velocity spectra findings, the likelihood repair site thrombosis is extremely low (<1%), as is the detection of >50% DR stenosis within 3 months of the procedure. A 2011 report from the Vascular Study Group of New England indicated only one-half of vascular surgeons routinely image carotid repairs with duplex ultrasound being the preferred technique [7]. Routine imaging was not associated with a reduction in operative strokes, but the incidence of restenosis was significantly less.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


