Intra-abdominal Vascular Trauma
NAM T. TRAN and AMELIA J. SIMPSON
Presentation
A 23-year-old patient is brought to the emergency department after a snowmobile crash where he was ejected and landed 100 feet from the vehicle. At the scene, the patient was unconscious and was intubated for airway protection. En route, the patient’s vital signs were BP of 80/45 and HR 130s. Initial trauma evaluation was remarkable for normal chest x-ray and lateral C-spine film. His pelvic AP film demonstrated a left pelvic ring fracture. Focused Assessment with Sonography in Trauma (FAST) exam was equivocal for intra-abdominal fluid. Given concern for pelvic bleeding, the patient was taken to the angiography suite. Pelvic angiogram did not show evidence of extravasation.
Differential Diagnosis
Abdominal vascular injuries are among the most lethal injuries in the trauma patient and are also some of the most difficult to deal with by the trauma surgeon. Typically, blunt injury to the abdomen results in injury to the solid organs such as spleen or liver. On the other hand, major vascular injury is seen in only 5% to 25% of patients admitted to the hospital with abdominal trauma. However, this is the most common cause of death in these patients. The high mortality associated with abdominal vascular trauma is multifactorial with the main reasons being massive acute blood loss, associated injuries, and difficulty with gaining rapid control of the injured bleeding vessel.
In our case presentation, the most likely diagnosis should be intra-abdominal hemorrhage. While this is most likely due to solid organ disruption, the possibility of vascular injury should not be excluded. Imaging modality such as multidetector CT can be especially helpful in blunt abdominal trauma. With the use of multiphasic image acquisition, one can not only differentiate arterial versus venous hemorrhage but also characterize areas of “contrast blush” as either contained or active hemorrhage. In recent years, advances in endovascular techniques have transition arteriography from just a diagnostic exam into a therapeutic treatment modality as well.
Case Continued
While in the angiography suite, the patient also underwent abdominal arteriography with the thought that one can embolize solid organ bleed if a contrast blush was present. Instead, the abdominal aortogram demonstrated extravasation from the perivisceral abdominal aorta (Fig. 1). The patient was then taken urgently to the operating room for definitive treatment.
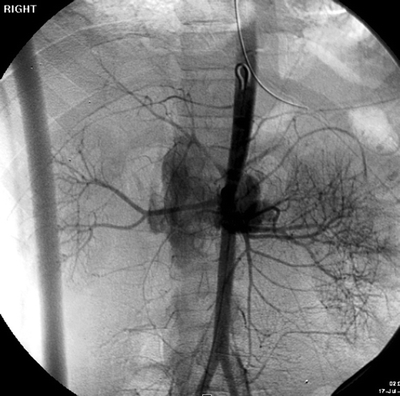
FIGURE 1 Abdominal aortogram demonstrating contrast blush from the para visceral aorta.
Operative Approach
The retroperitoneal space along with its vascular structures has been traditionally divided into three zones: zone I (midline), zone II (para median), and zone III (pelvic) (Fig. 2). From an anatomic surgical approach, these zones can be further divided into segments based on the retroperitoneal attachment of the mesentery. This facilitates the exposure and surgical management of vascular structures within each specific zone.
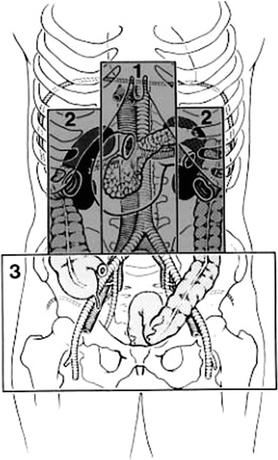
FIGURE 2 Zones of the abdomen retroperitoneal space. (From Selivanov V, et al. Mortality in retroperitoneal hematoma. J Trauma. 1984;24(12):1022–1027, with permission).
Zone I Supramesocolic
This space contains the suprarenal abdominal aorta, celiac trunk, superior mesenteric artery (SMA) and vein, and the origin of the renal vessels. Exposure of vascular structures in this zone is best accomplished via a left medial visceral rotation. In cases where rapid vascular control is required due to expanding hematoma or active hemorrhage, proximal control of the aorta should be obtained at the supraceliac aorta.
The left medial visceral rotation is best accomplished by initially dividing the retroperitoneal attachments along the line of Toldt and then reflecting the left colon, spleen, left kidney, tail of the pancreas, and gastric fundus to the midline (Fig. 3). With this exposure, the surgeon can visualize the entire abdominal aorta from the diaphragm to the aortic bifurcation. This maneuver, however, does take time and can potentially injure the spleen, left kidney, or left renal artery. Lastly, a critical step to gain proximal control of the aorta with the left medial visceral rotation is adequate division of the left crus so that a vascular clamp can be applied to the supra celiac aorta. Failure to fully mobilize the crus can lead to suboptimal proximal aortic control and ongoing hemorrhage.
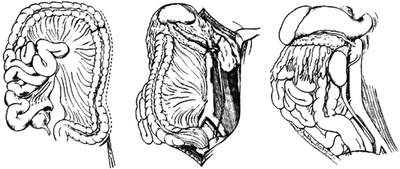
FIGURE 3 Left medial visceral rotation. (From Selivanov V, et al. Mortality in retroperitoneal hematoma. J Trauma. 1984;24(12):1022–1027, with permission).
Alternative approach to zone I supramesocolic vascular structure is an extended Kocher maneuver (Fig. 4). In this instance, the “C” loop of the duodenum and the head of the pancreas are elevated toward the midline. This approach will allow the surgeon to visualize the suprarenal aorta between the celiac axis and the SMA. However, one will not be able to gain control of the supra celiac aorta from this approach.
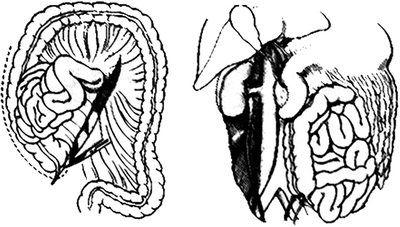
FIGURE 4 Extended Kocher maneuver. (From Selivanov V, et al. Mortality in retroperitoneal hematoma. J Trauma. 1984; 24(12):1022–1027, with permission).
Traditionally, one can gain access to the supra celiac aorta via the lesser sac. This is accomplished by dividing the gastrohepatic ligament, pushing the stomach to the left, and using one’s fingers to bluntly dissect around the crura of the supra celiac aorta. Placement of a nasogastric tube (NGT) can facilitate identification of the aorta especially in the severely hypotensive patient as the aorta may be flaccid; the aorta will be medial to the esophagus and the NGT as one tries to bluntly dissect in the region of the diaphragmatic hiatus. Lastly, the use of an aortic occlusion device or manual compression with one’s hand is an effective method of rapid aortic control at the diaphragm by direct application of pressure to compress the aorta against the spine.
Celiac Trunk
Dense neural and lymphatic tissues surround the celiac artery origin, thus making exposure of this area difficult especially in an actively bleeding patient. Typically, ligation is well tolerated for the left gastric artery, proximal splenic artery, or the common hepatic artery proximal to the origin of the gastroduodenal due to rich collateral networks in this area. Repair to the proper hepatic artery, due to its larger size, can be accomplished via lateral arteriorrhaphy, primary end-to-end anastomosis, or end-to-end bypass graft. Due to the high likelihood of associated hollow viscus injury and spillage, the use of prosthetic conduit is discouraged if arterial bypass is needed.
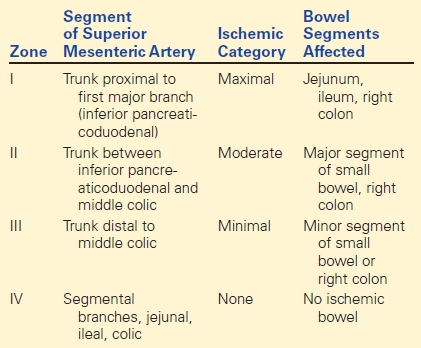
Superior Mesenteric Artery
Injury to the SMA is thankfully rare as mortality can be high with approximately one third of patients dying within 24 hours of presentation. Outcomes are related to the presence of hypotension, location of injury, presence of bowel infarction, and associated injuries. In 1972, Fullen and colleagues classified SMA injury into four zones with management of either ligation or repair dependent on the zone of injury (Table 1).
TABLE 1. Fullen’s Anatomic Classification of SMA Injury