OVERVIEW
The scope of interventional catheterization in children and adults with congenital heart disease has grown tremendously over the past 40–50 years. Advancements since the mid-1980s have made it possible to delay or, in some cases, avoid open heart surgery for many defects such as pulmonary or aortic valve stenosis, patent ductus arteriosus, and atrial septal defects. With the advent of the 21st century, transcatheter valve replacement of failed surgical valves has introduced exciting new treatment options for patients with congenital heart disease. This rapid progression of catheter-based interventions highlights the need for imaging modalities that complement the interventionalists’ use of traditional x-ray fluoroscopy to safely and effectively perform both simple and complex interventions.
The purpose of this chapter is to discuss the role of echocardiography in facilitating transcatheter interventions in congenital heart disease. In addition, the use of echocardiography in preintervention planning and postintervention evaluation will be briefly reviewed.
ECHOCARDIOGRAPHIC MODALITIES
Transthoracic (TTE), transesophageal (TEE), and intracardiac (ICE) echocardiography are each useful imaging options that have been well-described for use during cardiac catheterization. The details of image acquisition using these modalities are described elsewhere in this text and will not be repeated herein. However, the value of each modality will be discussed in the context of its advantages and limitations relative to other modalities in certain clinical situations. Table 36.1 provides a general summary of some of these advantages and disadvantages.
PRE- AND POSTPROCEDURE ECHOCARDIOGRAPHY
Because transthoracic echocardiography is the primary modality used to diagnose congenital heart defects, it is likewise the most commonly used modality for preintervention planning. In patients for whom TTE is inconclusive or incomplete in providing the necessary information to the interventionalist, TEE may provide better image quality and resolution, particularly of posterior structures and lesions. However, even with the best preprocedure imaging, some questions regarding the size, location, significance, or amenability of some lesions to intervention may only be answered at the time of catheterization.
Following transcatheter intervention, TTE is almost always the modality of choice for determining whether the lesion was appropriately treated. TTE is also used to assess for pericardial effusion that can result from vascular or myocardial perforation during the procedure.
COMMUNICATION BETWEEN IMAGER AND INTERVENTIONALIST
Except in situations using ICE where the interventionalist is also the echocardiographer, good communication between interventionalist and echocardiographer is vital. In such cases, the efficient and effective use of echocardiography for transcatheter interventions requires the participation of at least two individuals with different perspectives—one performing the procedure and another acquiring echo images. This arrangement works best when both parties have a consistent understanding of the patient’s anatomy and physiology. Furthermore, the information to be communicated between interventionalist and echocardiographer often changes over the course of the procedure, which can complicate the situation. Thus, it is important to use consistent, descriptive terminology that both individuals recognize. Generally this is best achieved by describing the specific lesions of interest both anatomically (e.g., anterior/posterior, superior/inferior, apical/basal) and relative to easily recognizable structures (e.g., atrioventricular valves, coronary sinus, aortic root, superior vena cava). Descriptors such as right, left, up, or down are confusing, as they can be understood in different ways depending upon one’s point of reference. If either person is unclear of what the other is describing, the procedure should be “paused” until they both have a clear understanding of the patient’s anatomy.
ATRIAL SEPTAL DEFECT AND PATENT FORAMEN OVALE (PFO) DEVICE CLOSURE
Device closure of ASD and PFO is one of the most common interventional procedures performed in the congenital catheterization lab. PFOs and most secundum ASDs can be closed safely from a transcatheter approach, obviating the need for surgical intervention. There are three currently available ASD closure devices: the AMPLATZER™ Septal Occluder (St. Jude Medical, St. Paul, MN, USA), the AMPLATZER™ “Cribriform” Multi-Fenestrated Septal Occluder (St. Jude Medical, St. Paul, MN, USA), and the GORE® HELEX® Septal Occluder (W.L. Gore and Associates, Newark, DE, USA).
Both the AMPLATZER™ Septal Occluder (ASO) and the Cribriform device (Fig. 36.1) consist of a semirigid nitinol wire outer frame with an inner Dacron® mesh layer. The outer frame has “memory,” meaning that it can be elongated and drawn into a delivery catheter and yet retain its configuration upon deployment. The configuration of the device is one of a “double-disk” design—a left atrial and a right atrial disk. While the Cribriform device has a narrow waist between the two disks, the ASO has a larger waist of variable diameter. The stated size of the Cribriform device (18, 25, and 35 mm) refers to the diameter of the right and left atrial disks. The size of the ASO (from 4 to 38 mm in varying increments) refers rather to the diameter of the central waist, which generally correlates with the measured size of the ASD being closed. Given the wide range of sizes available, the ASO can in principle be used to close very large defects. However, the presence of a very large ASD often reflects deficiencies in critical atrial septal rims needed to secure the device in place. AMPLATZER™ Septal Occluder devices are contraindicated if there is less than 5 mm of septal rim adjacent to certain anatomic structures: the right upper pulmonary vein, atrioventricular valves, coronary sinus, or inferior vena cava. While deficiency of the retroaortic rim is not an absolute contraindication to use of an AMPLATZER™ device, significant distortion of the aortic root by the device is discouraged.
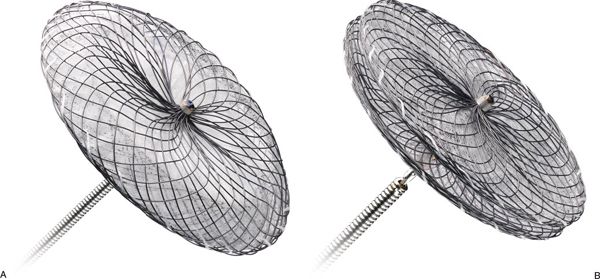
Figure 36.1. AMPLATZER™ Septal Occluder devices. A: AMPLATZER™ Septal Occluder device on a deployment cable. B: AMPLATZER™ Multi-Fenestrated (“Cribriform”) Septal Occluder device. (Courtesy of St. Jude Medical, St. Paul, MN, USA.)
The HELEX® device also has a “double-disk” design, but different structural characteristics than the AMPLATZER™ device (Fig. 36.2). This device consists of a single nitinol wire frame that loops upon itself during device deployment to a specific diameter (15–35 mm in 5-mm increments). As with the Cribriform device, there is a narrow, negligible central waist between the proximal (right atrial) and distal (left atrial) helical disks. The wire frame is covered in expanded polytetrafluoroethylene (ePTFE), a thin synthetic membrane that serves to occlude the defect. The HELEX® is lighter and more compliant than the AMPLATZER™ Occluder device, but is limited in the size of defects for which it can be used. In general, HELEX® devices can close defects whose maximum diameter is 50% of the device size, rounded up to the nearest millimeter. For example, a 20-mm HELEX® device can close up to a 10-mm defect; a 35-mm device can close up to an 18-mm defect. Unlike the AMPLATZER™ devices, there are no stated contraindications related to the absence of critical septal rims. However, caution should be taken so that the device does not disrupt or distort surrounding structures, such as the atrioventricular valves or aortic root.
Preintervention Echocardiographic Assessment
The role of echocardiography in the assessment of patients undergoing transcatheter closure of ASDs begins in the preprocedure phase with careful patient selection. The primary question to be answered is whether the ASD should be closed. Indications for device closure include evidence of significant left-to-right shunt (manifested by right ventricular enlargement), ischemic stroke suggesting right-to-left thromboembolism, and, in some cases, hypoxia with exertion (orthodeoxia-platypnea).
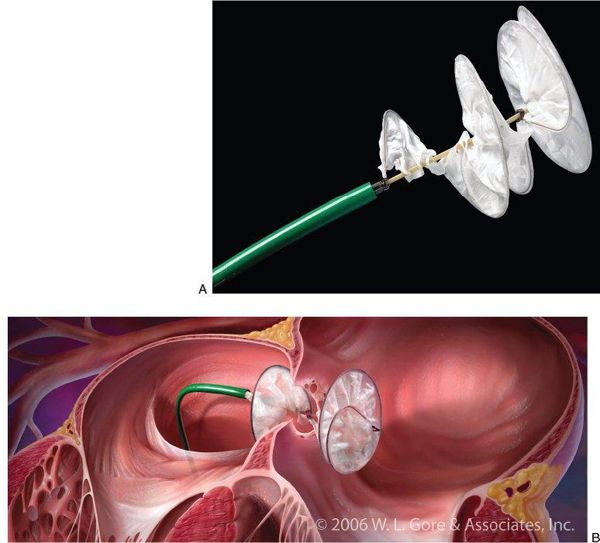
Figure 36.2. GORE® HELEX® Septal Occluder Device. A: Photo of the GORE® HELEX® Septal Occluder device attached to the delivery system. B: Schematic drawing of the HELEX® device in vivo during deployment across a fenestrated atrial septum. (Courtesy of W.L. Gore and Associates, Newark, DE, USA.)
In most cases, TTE is adequate to diagnose and characterize defects that may be amenable to device closure. As part of the preprocedural assessment, it is important to define that the defect is morphologically an ostium secundum defect. Primum, sinus venosus, and unroofed coronary sinus-type ASDs are not recommended for device closure. Precath TTE is also useful for estimating the number, size, and locations of the defect, which can be useful for anticipating which device could be used for closure. These factors are also important for anticipating risk of complications, such as device embolization or erosion, as well as the possibility of unsuccessful closure. As discussed above, the absence of critical septal rims precludes device closure of certain ASDs (Fig. 36.3, Video 36.1). This information facilitates the discussion of these risks with the patient and family at the time informed consent is obtained.
ASDs can be single or multiple and may coexist with a PFO. The presence of multiple defects does not preclude transcatheter device closure, but recognition of atrial septal fenestration may help the interventionalist anticipate which device(s) may be best suited for use. A fenestrated atrial septum with multiple defects proximal to one another may be closed with a single device. Multiple devices can be used to close fenestrations that may not be close enough to be covered by a single device (Fig. 36.4).
Complete echocardiographic evaluation prior to device closure of ASDs should include an identification of any coexisting structural or physiologic defects that may warrant a surgical approach (e.g., severe tricuspid valve regurgitation) or that may preclude ASD closure altogether (e.g., right-to-left shunting due to Eisenmenger syndrome). Of particular importance is the assessment of pulmonary venous connections. While partial anomalous pulmonary venous connection is most commonly associated with a sinus venosus-type ASD, it can also be seen with secundum defects. The presence of anomalous venous connections is usually an indication for surgical repair, although some cardiologists still advocate for device closure in the presence of a single anomalous pulmonary vein, attesting that a single anomalous vein does not produce a significant left-to-right volume load.
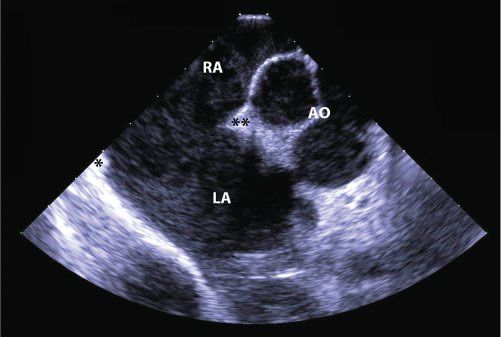
Figure 36.3. Large Secundum Atrial Septal Defect. Short-axis, intracardiac echocardiographic image of a very large secundum atrial septal defect which is not suitable for device closure. There is almost complete absence of the posterior rim (asterisk) but a reasonable retroaortic rim (asterisks). RA, right atrium; LA, left atrium; AO, aorta.
Diagnosis of a PFO is often difficult with standard TTE. Saline contrast injection with Valsalva release can elicit right-to-left shunting of contrast across the PFO that can be appreciated by TTE. With Valsalva release, right atrial pressure exceeds that of left atrial pressure and bubbles from agitated saline pass across the PFO into the left atrium. If TTE is inconclusive, TEE may be necessary. When used, TEE can provide a better anatomic description of the PFO, including whether it has a “tunnel-like” configuration that may make device closure somewhat more difficult.
Indications for closure of a PFO are more controversial. A PFO rarely results in significant shunt volume and ventricular enlargement. Most cardiologists, however, would offer device closure of a PFO if there is evidence of paradoxical embolism with stroke or TIA, or in the context of orthodeoxia-platypnea.
Intraprocedure Echocardiographic Assessment
Echocardiography during device closure of ASDs is extremely useful to confirm the size and location of the defect(s) and feasibility for closure. Depending upon patient size, clinical scenario, and institutional preference, transthoracic, transesophageal, or intracardiac echocardiography can each be used to facilitate device closure. For small children with good acoustic windows and simple ASDs, TTE is often an adequate imaging modality. Which modality is used is typically determined by individual or institutional preference. While TEE and ICE provide excellent image quality for facilitating device closure of ASDs, they each have unique advantages and disadvantages as previously discussed. Our institutional practice has been to use ICE almost exclusively during device closure of ASDs and PFOs.
The first objective of intraprocedure echocardiography is to confirm or correct the findings from preprocedure imaging. Pulmonary venous connections should be assessed by ICE or TEE (described in Chapter 35). The atrial septum should be interrogated, both with two-dimensional echocardiography and color Doppler (Fig. 36.5, Video 36.2). The size of the defect should be defined. The presence of additional fenestrations that may not have been appreciated on TTE should be described. The position of the defect(s) relative to other cardiac structures, such as the IVC, SVC, right upper pulmonary vein, atrioventricular valves, and aortic root should also be detailed. Often “balloon sizing” is used as another method of measuring the size of the defect. With this technique, a soft sizing balloon is passed over a wire across the defect and inflated with dilute radiopaque contrast. As long as the diameter of the balloon at full inflation is larger than the defect size, there will be a distinct waist in the balloon. The balloon is deflated just until color Doppler demonstrates flow through the defect around the balloon. It is then inflated again slightly, just until the flow disappears. The waist of the balloon can be measured by fluoroscopically and by 2D echocardiography. The measurements obtained are then used for selecting the appropriate device type and size for defect closure.
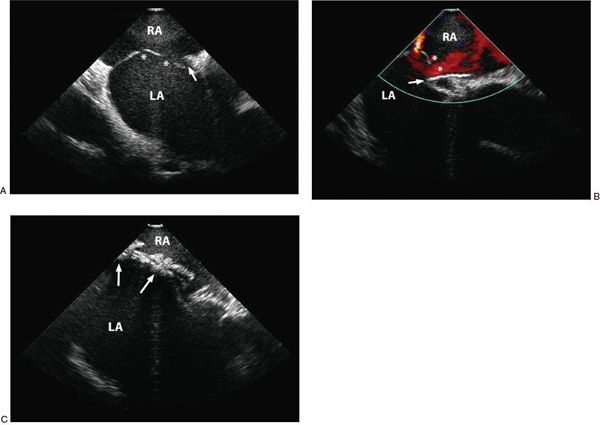
Figure 36.4. Fenestrated Atrial Septum. A, Two-dimensional intracardiac echocardiographic image of multiple defects (asterisk) and a patent foramen ovale (arrow). B: Color flow Doppler showing multiple residual defects (asterisk) during initial attempted closure with a single GORE® HELEX® device (arrow). C: The fenestrations are ultimately closed using two HELEX® devices (arrows). RA, right atrium; LA, left atrium.
ICE or TEE can also provide useful insight into the morphology of PFOs. A PFO with a short segment of overlap between limbus and valve of the fossa ovalis can usually be closed quickly and easily. A longer “tunnel-like” overlap may require more careful device selection or alternative approaches for optimal closure (Fig. 36.6). Balloon sizing is usually not needed, but can be used if a better understanding of the length of the PFO tunnel is desired.
After anatomic evaluation of the defect, echocardiography is used to help with positioning and deployment of the device. Both TEE and ICE provide real-time imaging as the delivery system is advanced across the ASD or PFO. The distal disk of the device is advanced out of the deployment catheter. Echo imaging can be helpful to ensure that the distal disk is in the body of the left atrium, rather than abutting the free wall or in the pulmonary vein or appendage. The device-catheter system is withdrawn until the left atrial disk just abuts the atrial septum, at which time the right atrial disk is exposed. The device at this point is not yet released and can be withdrawn and repositioned or removed if necessary. Echocardiographic imaging confirms appropriate placement of the device and its relationship to surrounding structures. The presence of septal tissue between the two disks should be documented from multiple orthogonal views. In the setting of a deficient retroaortic rim, the disks should be shown to straddle the aortic root without significantly distorting it. Disruption of the integrity of the aortic root may manifest as increased aortic valve regurgitation. Further, the proximity of the device to the mitral and tricuspid valves and the inferior and superior venae cavae should be clearly defined.
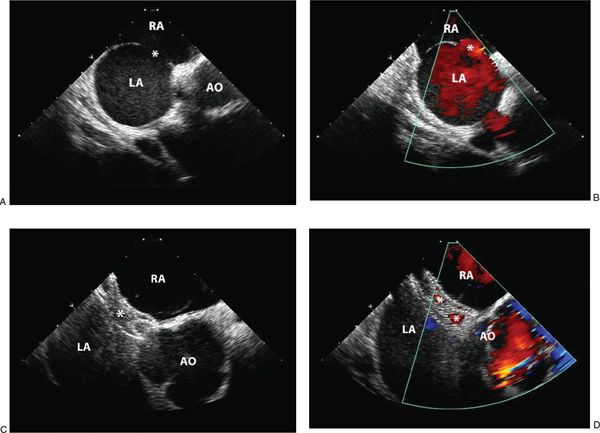
Figure 36.5. Secundum Atrial Septal Defect. A: Two-dimensional intracardiac echocardiographic image of a single secundum atrial septal defect (asterisk) with adequate septal rims for device closure. B: Color flow Doppler demonstrating left-to-right shunting through the defect (asterisk). C: Imaging of an AMPLATZER™ Septal Occluder device (asterisk) before device release demonstrates appropriate device position. D: Following device release, color flow Doppler demonstrates small residual shunt through the device, which should close with time. RA, right atrium; LA, left atrium; AO, aorta.
Additionally, the device should be interrogated along its length to look for a residual shunt. Frequently, residual shunts around the device or through the device (in the case of AMPLATZER™ devices) can be appreciated immediately after device placement. Flow through AMPLATZER ™ devices is a normal phenomenon and does not indicate unsuccessful or incomplete closure (Video 36.3). Such residual shunts should resolve with endothelialization of the device. Shunts around the device often resolve over time, as well. However, some degree of shunting may persist, and is more commonly seen with HELEX® devices. This presents a challenging question of whether to reattempt closure with a different device or to allow the residual defect to close over time. In some cases, removal of the first device and closure with a larger device or placement of a second closure device may be warranted.
Upon release the orientation of the device may change, assuming a more “anatomic” position. At this point, it should be reassessed echocardiographically to confirm appropriate and secure position. Any residual shunt should also be documented.
Postprocedure Echocardiographic Assessment
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


