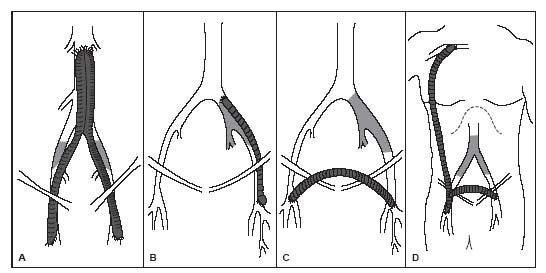
The infrarenal abdominal aorta and iliac arteries are among the most common sites of chronic obliterative atherosclerosis, accounting for about one third of all peripheral artery disease (PAD) cases. The standard surgical treatment for aortoiliac disease is aortobifemoral bypass, where a Y-shaped graft is attached to the distal abdominal aorta proximally, and each limb of the Y-graft is attached to the common femoral artery (CFA) distally (Fig. 15.1). Occasionally, the distal limb(s) may be anastomosed to the profunda femoral artery (PFA) when the CFA is diseased. Less common surgical procedures for aortoiliac disease include aortoiliac endarterectomy for the treatment of focal iliac disease, and axillofemoral bypass for patients with occlusive disease of the distal abdominal aorta and severe co-morbidities (Fig. 15.1). In patients with unilateral iliac disease, additional surgical options include femorofemoral crossover bypass or iliofemoral bypass (Fig. 15.1).
With respect to aortobifemoral bypass, a meta-analysis of 23 series including over 8000 patients operated between 1975 and 1995 reported an aggregated operative mortality of 4.6% in early studies (performed in the 1970s) and 3.3% in later studies (in the 1990s) (1). The aggregated major morbidity was 13% and 8% for each time period, respectively. Limb-based patency rates for patients with claudication were 91% and 87% at 5 and 10 years, respectively; the corresponding patency rates in patients with critical limb ischemia were 87% and 82%, respectively. No difference in patency was detected between older and more recent studies. Aortoiliac endarterectomy has been associated with patency rates in modern series ranging from 88% to 94% at 1 year and from 60% to 80% at 5 years (2,3). However, since best endarterectomy results were obtained in patients with focal iliac disease, this technique has been largely replaced by endovascular intervention. Axillofemoral bypass has been performed with patency rates ranging from 78% to 93% at 1 year and from 50% to 80% at 5 years (4).
This chapter focuses on percutaneous intervention of aortoiliac disease, which has become the treatment of choice for many patients with aortoiliac atherosclerotic disease.
ANATOMY
The iliac vessels originate from the distal abdominal aorta (Fig. 15.2). The common iliac artery (CIA) subsequently divides into the internal iliac artery (IIA) supplying the pelvic organs, and the external iliac artery (EIA), which continues to become CFA at the level of the inguinal ligament. The diameters of the CIA and the EIA range from 8 to 10 mm, and from 6 to 8 mm, respectively. Because of the abundant collateral circulation through the inferior mesenteric artery, lumbar vessels, and the IIA, limb-threatening ischemia is rare, even in the presence of total occlusion of distal abdominal aorta as long as there is no associated severe femoropopliteal or tibial disease.
Three distinct patterns of atherosclerotic involvement of the infrarenal aorta and the iliac vessels have been described (Table 15.1 and Fig. 15.3) (5). Type I refers to exclusive involvement of the distal abdominal aorta and the CIA, is present in about 5% to 10% of patients with PAD, and is more frequently encountered among women. Type II involves the infrarenal aorta, CIA, and EIA and may extend into the CFA; this pattern may be observed in 35% of patients with PAD. Type III is the most common pattern and involves the infrarenal aorta, iliac, femoral, and popliteal arteries as well as the infrapopliteal circulation.
PATIENT SELECTION
Patients with aortoiliac occlusive disease present most frequently with lifestyle-limiting claudication. Whereas calf pain on exertion is the leading symptom in femoropopliteal arterial disease, patients with aortoiliac involvement may have less characteristic symptoms such as ambulatory back, buttock, hip, and thigh pain, in addition to calf pain. These conditions are frequently misinterpreted as representing pathology of the lower back or hip, delaying diagnosis for many years. Since the chronic progressive nature of the disease allows for the development of a robust collateral circulation, associated critical limb ischemia is rare provided the femoropopliteal and tibial vessels are patent. This is true even in the presence of a distal aortic occlusion (Leriche syndrome).
The most important part of the physical exam in a patient with suspected iliac disease includes an assessment of the CFA pulses. An absent CFA pulse usually indicates either an occlusion of the ipsilateral iliac or CFA (Figure 15.4). The routine diagnostic workup of patients with suspected aortoiliac includes a hemodynamic evaluation (including ankle–brachial index [ABI], segmental limb pressures [SLP], pulse volume recordings [PVR], and exercise testing). The typical hemodynamic fi ndings in patients with iliac disease include a decreased ABI and a significant decrement in the SLP between the brachial artery and upper thigh pressure (i.e., diminished thigh–brachial index) that is associated with a drop in the amplitude of the PVR between the brachial artery and upper thigh. Exercise testing is particularly useful in patients with typical claudication symptoms suggestive of iliac disease (e.g., buttock claudication) but with a normal resting hemodynamic assessment.
Figure 15.1 • Surgical bypass for aorto-iliac disease. A: Aorto-bifemoralbypass. B: Ilio-femoral bypass. C: Femoral-femoral bypass. D: Axillo-femoral bypass combined with femoral-femoral bypass. (Adapted from Neville RF, Deaton, DH. Lower extremity revascularization. Endovascular Today, February 2004:49–56.)
Duplex ultrasound imaging is helpful for patients in whom adequate visualization of the iliac arteries is possible. In cases with suboptimal visualization of the pelvic region, assessment of the Doppler flow analysis in the CFA may offer useful information. The presence of a physiologic triphasic waveform in the CFA makes a hemodynamically relevant iliac artery stenosis unlikely.
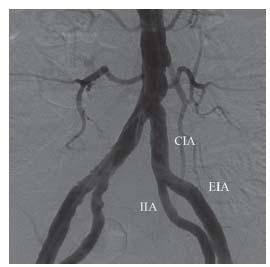
Figure 15.2 • Normal anatomy of the distal abdominal aorta, the common iliac arteries (CIA), the internal iliac arteries (IIA), and the external iliac arteries (EIA).
Anatomic assessment of the aortoiliac vessels with computed tomography angiography (CTA) or magnetic resonance angiography (MRA) is highly effective. In addition to having high rates of sensitivity and specifi city for the diagnosis of disease, these studies are invaluable in providing information to help guide percutaneous revascularization, if indicated.
An iliofemoral stenosis is generally considered hemodynamically significant if the luminal stenosis on angiography is ≥70% (6). In borderline cases, assessment of the translesional pressure gradient may be helpful. Although clear-cut pressure gradient thresholds to defi ne the hemodynamic relevance have not been identifi ed, a systolic peak gradient ≥ 10 mm Hg prevasodilation and >15 mm Hg postvasodilatation—as measured with a 4- or 5-Fr. diagnostic catheter—is generally considered significant (7). Appropriate patient selection, taking into consideration the clinical status of the patient, the location, morphology, and physiologic signifi cance of the aortoiliac lesion, as well as the operator’s experience, are key factors in determining the appropriateness of endovascular intervention.
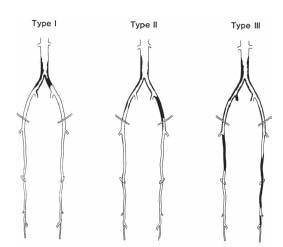
Figure 15.3 • Typical patterns of atherosclerotic involvement of the infrarenal aorta and the iliac vessels, as described also in Table 15.1. (Reproduced with permission from Brewster DC. Clinical and anatomical considerations for surgery in aortoiliac disease and results of surgical treatment. Circulation. 1991;83(2 suppl):142–152.)
The TransAtlantic Inter-Society Consensus (TASC) Working Group published in the year 2000 a comprehensive review of the management of PAD that included classifi cation and management guidelines also of iliac disease (4). Recently, these guidelines and defi nitions were updated with a new TASC II classifi cation for aortoiliac disease (Fig. 15.5) (7). According to the document, for simple lesions (TASC A–B lesions), endovascular treatment is the therapy of choice and for very complex disease (TASC D lesions), surgery is recommended. There was a lack of consensus regarding TASC C lesions. For this lesion subset, the decision should be made on a case-by-case basis, depending on the clinical presentation, patient comorbidities, the lesion morphology, and operator experience. Despite these general recommendations, the lack of randomized data comparing surgical versus endovascular revascularization and the continuous evolution in revascularization technique and equipment have resulted in marked variation in treatment strategies across institutions. In experienced centers, it is common to adopt an endovascular first approach to even complex aortoiliac disease and to refer only those patients who either fail this endovascular approach or who cannot be approached using endovascular techniques (e.g., complex aneurismal disease, significant CFA disease) to surgery (8–10).
DIAGNOSTIC ANGIOGRAPHY
Access
Generally, diagnostic angiography of the aortoiliac region is performed using retrograde CFA access contralateral to the limb that is more symptomatic and/or demonstrates the worse hemodynamic compromise. If imaging studies (i.e., duplex ultrasound, CTA, or MRA) have already been performed, access can be chosen based on the anatomic fi ndings of these studies. In this circumstance, the location of the lesion(s) to be treated is the most important factor determining the access site. For example, EIA stenoses—especially if located in the distal segment—are treated using a crossover approach from the contralateral CFA, whereas CIA stenoses are typically approached using ipsilateral retrograde CFA access. The choice of access site(s) for the treatment of iliac occlusions requires careful consideration, with some cases requiring bilateral CFA access, and others requiring brachial access, depending on the specifi c anatomy (see below).
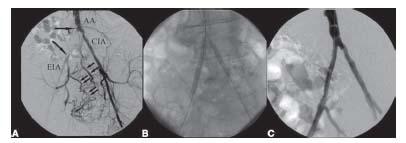
Figure 15.4 • Interventional treatment of right common iliac artery (CIA) occlusion (delineated by large arrows) using antegrade approach. A: Baseline pelvic angiography using left brachial access demonstrating occlusion of right CIA and severe stenosis at origin of left CIA. Small arrows demonstrate collateral fi lling of the right internal iliac artery and subsequently of the right external iliac artery (EIA). AA, abdominal aorta. B: Kissing stenting of the aortic bifurcation was performed using dual arterial access from the left brachial artery to treat the right CIA (note the tip of the 90-cm sheath at the aortic bifurcation) and retrograde left common femoral artery access to treat the left CIA stenosis. C: Final angiographic result after kissing stenting.
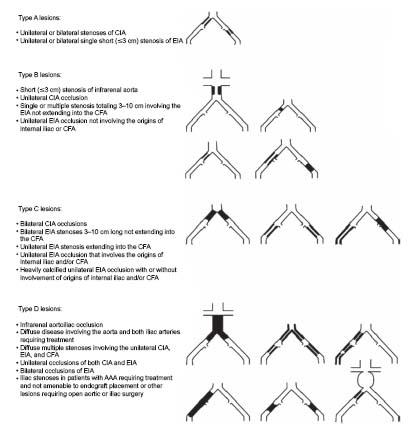
Figure 15.5 • TransAtlantic Inter-Society Consensus (TASC II) morphologic stratifi cation of iliac lesions. (Reprinted from Norgren L, Hiatt WR, Dormandy JA, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vase Surg. 2007;45(suppl S):S5–S67, with permission from Elsevier.)
Views and Catheters
significant tortuosity of the iliac arteries and eccentricity of atherosclerotic involvement in the aortoiliac region is frequent. Therefore, angulated views (30° to 35° right anterior oblique [RAO] and 30° to 35° left anterior oblique [LAO]) are important to optimize the sensitivity of the study. The LAO view will typically provide optimal visualization of the origin of the right CIA and the right CIA bifurcation, whereas the RAO view provides optimal visualization of the left CIA and the left CIA bifurcation. Conversely, the LAO view provides for optimal visualization of the distal left EIA and the left CFA bifurcation and vice versa for the RAO view. In most cases, pelvic diagnostic angiography can be performed using 5- or 6-Fr. pigtail catheter. The catheter is typically positioned at the level of L3, and injection of contrast at 10 to 15 cc/sec for a total of 20 to 30 cc using a mechanical assist device and digital subtraction angiography (DSA) will allow for adequate visualization of the aortoiliac region.
INTERVENTION
Antiplatelet Agents and Anticoagulants
Patients undergoing planned revascularization should preferably be on aspirin for a minimum of 48 hours prior to the procedure. Alternatively, 325-mg oral aspirin may be given prior to gaining femoral access. There is no consensus regarding the use of preprocedural clopidogrel for iliac intervention. However, this practice should be avoided in the treatment of iliac occlusions, where the use of preprocedural clopidogrel might complicate surgical bailout of a significant complication (e.g., perforation). Following successful iliac stenting, the authors’ practice is to administer clopidogrel 75 mg/day for at least 1 month, in addition to lifelong aspirin. However, it should be highlighted that in some centers, clopidogrel is not prescribed, especially in the presence of large iliac vessels or in patients at high risk for bleeding events.
The optimal type and level of anticoagulation for peripheral intervention (including aortoiliac intervention) has not been defined. Most investigators use unfractionated heparin for aortoiliac intervention and aim for an activated clotting time (ACT) of between 200 and 250 seconds. Non-Food and Drug Administration (FDA)-approved alternative anticoagulation agents include direct thrombin inhibitors such as bivalirudin or low-molecular-weight heparins. The use of the latter agents, which do not have a reversing agent available, is discouraged for the treatment of iliac occlusive disease, due to the risk of perforation during these procedures.
Interventional Treatment of Aortoiliac Stenotic Disease
In the current era, the interventional treatment of stenoses in the aortoiliac territory is usually straightforward for appropriately trained endovascular specialists, with a procedural success approaching 100%. These stenoses are easily crossed using 0.035″; wires and treated using 0.035″; balloon and stent systems. Although compelling data supporting stenting in all cases are lacking, most operators have adopted the practice of stenting all iliac lesions because of the predictability of the procedural result achieved with iliac stenting. The major considerations in the interventional strategy in treating stenotic disease of the aortoiliac territory relates to the location of the disease.
OSTIUM OF THE COMMON ILIAC ARTERY AND AORTIC BIFURCATION
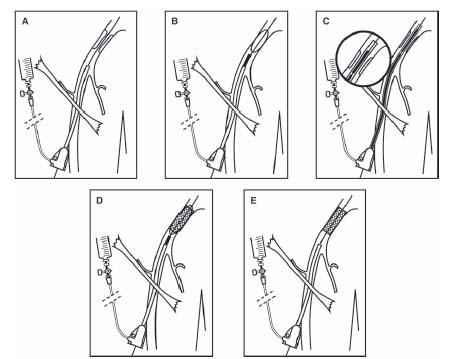
Unilateral ostial lesions of the CIA are best approached using ipsilateral retrograde CFA access. In most circumstances, the authors’ preference is to perform kissing angioplasty/stenting of the aortoiliac bifurcation, with the goal of preventing plaque shift into the contralateral CIA at the time of stent deployment, which will require bilateral retrograde CFA access. Disease of the aortic bifurcation requires a kissing angioplasty/stenting technique in all cases, using bilateral retrograde femoral access. Stenting at the aortoiliac bifurcation is typically performed using balloon-expandable stents, due to their greater radial strength and ease of accurate positioning (Fig. 15.3). Based on the caliber of most balloon-expandable stents used to treat the typical caliber of the CIA (i.e., 7 to 10 mm), a 7 Fr. is required for most cases.
Following sheath placement, 0.035″; wire(s) (e.g., Wholey, SupraCore) are advanced across one or both CIA into the abdominal aorta. Predilation with an undersized noncompliant balloon is recommended prior to stenting. Stent placement should be performed under road map guidance, using either a pigtail in the distal abdominal aorta or injection through the sidearm of longer CFA sheaths (i.e., 23 cm) prior to insertion of the stent(s) through the sheath(s). With kissing stenting of the aortoiliac bifurcation, the stents are positioned to extend into the distal abdominal aorta. The degree to which the stents are positioned above the aortoiliac bifurcation depends largely on the presence of disease in the distal abdominal aorta, with the goal of covering significant distal aortic disease. In the absence of disease, it is typical to extend the stents about 5 mm above the true bifurcation. Raising the aortic bifurcation in this way clearly compromises and may preclude future attempts at contralateral access. However, one should rarely compromise on the appropriate stent placement based on this concern.
The choice of stent diameter during kissing stenting is an important consideration. This is determined by the diameter of the ipsilateral CIA and the diameter of the distal abdominal aorta. The combined diameter of two circular stents placed in each CIA extending into the distal abdominal aorta is estimated as follows: 0.8(D1 + D2)=D3 (where D1 and D2 represent the effective diameter of both CIA stents, and D3 represents the diameter of both stents in the distal abdominal aorta). When the distal abdominal aorta is relatively small, the diameter of the CIA stents may be purposely undersized to minimize the risk of aortic rupture. The only circumstance in which the authors have used self-expanding stents at the aortoiliac bifurcation is where both common iliac arteries are of large caliber and the distal abdominal aorta is of insufficient caliber to accommodate the combined diameter of both kissing stents (Fig. 15.6).
NONOSTIAL COMMON ILIAC ARTERY AND PROXIMA L EXTERNAL ILIAC ARTERY
Stenoses of the CIA (excluding the ostium) and the proximal EIA are usually approached using ipsilateral retrograde CFA access (Fig. 15.7). Stenting in these locations may be performed using either balloon- or self-expanding stents. The major determinant of stent choice in this location is related to variations in vessel diameter. For disease with an equivalent proximal and distal reference diameter, a balloon-expandable stent may be used. However, where there is a significant size mismatch between the proximal and distal reference segments, a self-expanding stent is more appropriate, with the stent being sized to be 1 to 2 mm larger than the largest reference diameter.
DISTAL EXTERNAL ILIAC ARTERY STENOSES
In the presence of a stenosis located in the distal portion of the EIA artery, the ipsilateral retrograde CFA approach should not be used due to the immediate proximity between the access site and the lesion. Instead, access should be gained using a crossover technique from the contralateral CFA access site (Figs. 15.8 and 15.9. Stenting in this location is typically performed using self-expanding stents, as the distal EIA is in close proximity to the hip joint and is likely subject to significant conformational changes increasing the risk of stent failure with balloon-expandable stents in this location.
Interventional Treatment of Iliac Artery Occlusions
The interventional treatment of occlusions in the aortoiliac territory presents a fundamentally different set of challenges compared with the treatment of stenoses (Figs. 15.10 and 15.11). The choice and technique of access is more challenging, with a greater requirement for upper extremity access, and dual retrograde CFA access or dual upper extremity and unilateral retrograde CFA access. Often, the CFA distal to the iliac occlusion will need to be accessed, increasing the challenge of gaining successful CFA access.
One of the primary questions that should be addressed prior to attempting the treatment of an iliac occlusion is whether one wishes to approach the occlusion using an antegrade or retrograde approach. The approach will usually be dictated by the anatomy of the occlusion. For example, a fl ush occlusion of the CIA will dictate a retrograde approach, whereas an occlusion of the CIA with a proximal stump will allow either an antegrade or retrograde approach. The antegrade approach has the potential advantage of minimizing the risk of dissection or perforation of the distal abdominal aorta. However, in the authors’ experience, this has not been a significant issue in practice, and there are times when the retrograde approach is technically more straightforward and expedient.
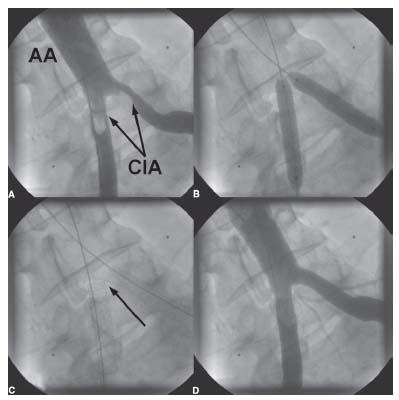
Figure 15.6 • Interventional treatment of aortoiliac bifurcation disease. A: Baseline pelvic angiogram demonstrating significant stenosis of the ostia of both common iliac arteries (CIA) (arrows). AA, abdominal aorta. B: Bilateral retrograde CFA access was obtained and 0.035″ wires were advanced into the abdominal aorta from each CFA access site. Simultaneous kissing stenting of both common iliac artery ostia was performed. C: The stents were purposely not placed in the distal abdominal aorta due to the absence of plaque in that location. This does allow for future crossover maneuvers. The location of the carina of the CIA stents is indicated by the arrow. D: Final angiographic result.
When using an antegrade approach to the treatment of an iliac occlusion, brachial access or contralateral CFA access is required. Using brachial access for these cases is challenging and should be reserved for experienced interventionalists. The left brachial access is preferred because it allows for a more direct access to the descending aorta (i.e., shorter length) and at the same time it minimizes the risk of cerebral embolization since the only cerebral vessel that is crossed is the left vertebral artery. The brachial artery should be punctured in its distal part above the antecubital fossa, where effective hemostasis may be achieved by arterial compression against the humerus. Following initial placement of a short sheath in the left brachial artery, a 0.035″ wire is delivered to the distal abdominal aorta, which is then used to exchange the short sheath for a long 6-Fr. Shuttle sheath whose tip is positioned in the distal abdominal aorta. This approach has the advantage of allowing for a more coaxial alignment of the sheath with the occlusion that improves the ability to cross the occlusion and deliver equipment. The major shortcomings of this approach include the need to manage the brachial access carefully (i.e., monitoring for ischemic complications). In addition, the long length between the brachial artery and the site of the occlusion may sometimes compromise the pushability of wires and catheters, making it difficult to cross complex calcifi ed occlusions. Finally, treating distal EIA disease in tall individuals may not be possible from the brachial access site due to limitations in the length of balloon and stent delivery catheters.
When using retrograde contralateral CFA access to cross an iliac occlusion in the antegrade direction, there are two broad scenarios that merit description:
1. Treatment of a CIA or CIA/EIA occlusion. In this instance, the authors typically use a Simmons 1 or 2 catheter to engage the contralateral CIA. These catheters should typically be shaped in the thoracic aorta and are withdrawn carefully to engage the contralateral CIA. A 0.035″ glidewire is then used to cross the occlusion that is snared from below using retrograde CFA access. Angioplasty and stenting are then typically performed using the retrograde approach.
2. Treatment of an EIA occlusion (Fig. 15.12). In this instance, a 7-Fr. contralateral sheath can be placed with its tip in the distal CIA, proximal to the EIA occlusion. The authors will use the ipsilateral IIA to provide wire purchase for delivery of the contralateral sheath. Once the contralateral sheath is in position, the occlusion is crossed using 0.035″ glidewires and 0.035″ supportive catheters (e.g., glide catheter, Bernstein catheter). Once the wire is in the CFA (using a re-entry device if required), subsequent angioplasty and stenting can be performed using the antegrade approach.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree