Internal Carotid Artery Fibromuscular Dysplasia
MARY E. JENSEN and AVERY J. EVANS
Presentation
A 49-year-old woman presented to her primary care physician after a 5-minute episode of left amaurosis fugax, followed by a headache. She was initially seen by an ophthalmologist who noted a normal eye exam with intact visual fields, but identified a left carotid bruit. Her past medical history was important for sicca syndrome, hyperlipidemia, and mitral valve prolapse (MVP). She was taking pravastatin 40 mg qd at the time of the event; afterward, aspirin, 325 mg qd, was added to her medications. She was a nonsmoker, had no family history of cardiovascular disease, and had no personal history of hypertension. She denied any recurrent visual changes, neurologic abnormalities, or history of headaches. At physical examination, her vital signs and neurologic exam were normal. She had bilateral carotid bruits, left greater than right. A known left-sided thyroid nodule was stable.
Differential Diagnosis
The differential diagnosis of amaurosis fugax is extensive and can be divided into circulatory, ocular, and neurologic etiologies (Table 1). This patient’s history of MVP, hypercholesterolemia, sicca syndrome (also known as Sjögren’s syndrome) (SS), and a headache associated with the visual disturbance give rise to several potential diagnoses (Table 1).
TABLE 1. Causes of Amaurosis Fugax

The presence of a bruit, and the unilateral nature and duration of the visual loss, are indicative of an embolic etiology from a carotid stenosis, most likely from bifurcation disease. This patient’s history of hypercholesterolemia puts her at risk for atherosclerotic disease, but she is young for advanced disease and has no other risk factors. Carotid artery dissection is a strong consideration, particularly when headache and/or neck pain is reported or Horner’s syndrome symptoms (ptosis, miosis, anhidrosis, and enophthalmos) are present. Visual disturbance or loss from a carotid dissection may be from decreased retinal perfusion caused by low flow, or from thrombus forming on the dissection flap or in the false lumen, which embolizes to the retinal artery or to portions of the brain that supply the optic radiations. Intrinsic vascular diseases that result in turbulent flow or stenosis, such as fibromuscular dysplasia (FMD) or large vessel arteritides, are other possibilities.
With the patient’s history of MVP, a cardiac embolic source must be excluded. SS is a chronic autoimmune disease in which the exocrine glands are damaged or destroyed. Eye dryness may result in blurry vision but not visual loss unless the cornea is injured. However, inflammatory vasculitis involving small vessels is associated with SS and can involve the brain and cranial nerves. Lastly, visual disturbance, usually binocular, is common with migrainous disease, but this patient had no history of chronic headaches.
Workup
Carotid Dopplers were negative for bifurcation plaque, and a transthoracic echocardiogram (TTE) did not demonstrate valve vegetations or mural thrombus. Since cardiac and carotid bifurcation disease were excluded by sonography as potential embolic sources, the next step was to evaluate those portions of the carotid artery that are difficult to see on carotid ultrasound; that is, the aortic arch and brachiocephalic origins, and the retromandibular segments.
Computed tomographic angiography (CTA) (Fig. 1A and B) showed bilateral internal carotid artery (ICA) irregularities consisting of concentric and eccentric plications with intervening saccular outpouchings (“string of beads”) and a focal dissection with an expanded lumen on the left (Fig. 1A). The diseased segments extended from the C1-C2 level to the skull base. The diagnosis of bilateral ICA FMD was made based upon the noninvasive imaging findings and confirmed by cervicocerebral angiography (Fig. 2), where no flow limitation or thrombus formation was noted. Also, angiographic evaluation of the renal arteries at the same time showed mild-to-moderate FMD.
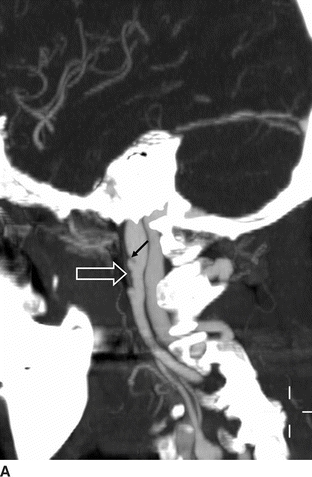
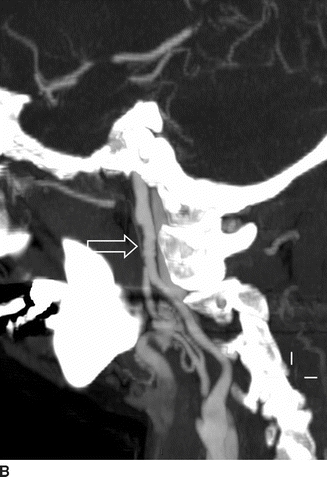
FIGURE 1 Sagittal maximum intensity projection (MIP) images from computed tomographic angiography (CTA) of the neck (A and B) show bilateral internal carotid artery (ICA) short-segment irregularities consisting of concentric and eccentric plications with intervening saccular outpouchings (“string of beads”) (open arrows) at the C1-C2 level. On the left, there is a focal dissection with visualization of the flap (arrow, A) and expansion of the lumen distal to the flap to the carotid canal.
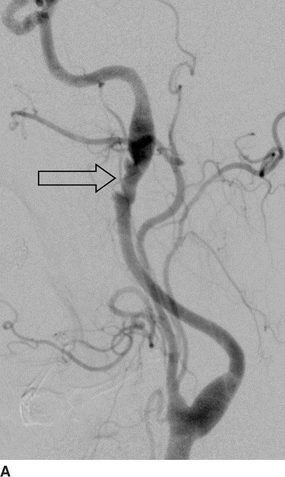
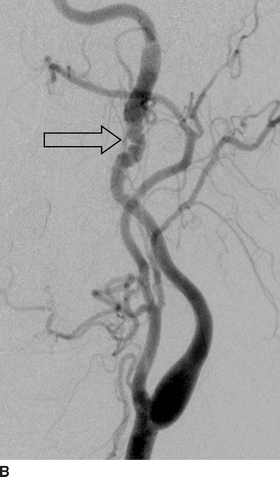
FIGURE 2 Lateral images from distal subtraction angiography (DSA) of the left (A) and right (B) common carotid bifurcations confirm the abnormalities of the distal ICAs. The “string of beads” is better seen on the DSA (open arrows) and shows the intervening areas of stricture and dilatation, particularly on the right. Again noted is the dissection flap on the left with distal dilatation of the vessel. The right ICA also shows mild dilatation of the vessel proximal to the skull base.
Diagnosis of Cerebrovascular FMD
This patient’s radiographic studies demonstrated the classic appearance of FMD, with the focal areas of dilatation and constriction involving the distal ICAs, evidence of a dissection flap, variable caliber of the diseased segments compared to the normal ICA, and similar involvement of the renal arteries.
Prevalence
FMD is a noninflammatory, nonatherosclerotic vascular disease originally described in 1938 by Ledbetter and Bergland and labeled as “fibromuscular hyperplasia” by McCormack et al. in 1958. The prevalence of FMD in the population is unknown, but it is more common in women than in men by a ratio of 9:1. The U.S. Registry for FMD published its initial results in 447 patients and found the incidence of renal and cerebrovascular FMD in their participants to be nearly equal, contradicting previous data that found the incidence of renal FMD greater than cerebrovascular FMD by almost 2:1. The prevalence of cerebrovascular FMD has been reported as ranging from 0.3% to 3.2% based upon consecutive cerebral angiographic studies. The cause of FMD is also unknown, and although hormonal factors have been proposed, none have been proven. Evidence supports a genetic susceptibility to FMD, and two small studies suggest a relationship between cigarette smoking and the risk of FMD.
Pathology
In 1971, Harrison and McCormack published a paper on the classification of FMD, dividing lesions into medial, intimal, and adventitial/periarterial types, according to the arterial layer most affected. The medial variant is the most common, accounting for greater than 90% of all cases. It is characterized by deposition of loose collagen in zones of degenerating elastic fibrils, resulting in fibromuscular ridges causing circumferential stenoses, with intervening areas of smooth muscle loss and subsequent arterial dilatation. The result is the “string of beads” appearance on imaging, which is found primarily in the mid-to-distal portions of the internal carotid and vertebral arteries.
Intimal FMD accounts for 1% to 2% of all cases and is caused by accumulation of fibrous tissue in the intima with preservation and reduplication of the internal elastic lamina, resulting in focal or tubular angiographic stenoses.
Adventitial FMD is seen in less than 1% of cases and shows collagen deposition in the adventitia with extension into the periarterial tissue and focal infiltration of lymphocytes. Tubular stenosis is the most common radiographic appearance.
Clinical Presentation
The clinical manifestations of cerebrovascular FMD are highly variable and often nonspecific. Data from 447 patients enrolled in the U.S. Registry for FMD show the most common symptom is headache (60%), often of the migrainous variety (50%). Pulsatile tinnitus or a “swooshing” sound was described by 27.5% of participants, followed by neck pain and dizziness/lightheadedness (20% to 26%). Cervical bruit was the presenting sign in 22% of patients; reported neurologic events included hemispheric TIA (13.4%), cervical artery dissection (12.1%), completed stroke (9.8%), and amaurosis fugax (5.2%).
FMD is associated with carotid dissection and intracranial aneurysm. It has been estimated that 15% to 20% of cervical artery dissections are FMD related. In the U.S. Registry, dissections at any location were noted in 19.7%, 20% of those patients had multiple dissections, dissections were more common in males, and the most common location was the carotid artery, followed by the renal artery and the vertebral artery. In the U.S. Registry, 17% of participants reported an aneurysm at any location, with the prevalence of asymptomatic intracranial aneurysms reaching 7.3%.
Differential Diagnosis
Standing Waves
A pattern of “ripples” may be seen in a vessel downstream from the catheter tip during contrast injection. These undulations are concentric and regularly spaced, with no intervening areas of stricture or dilatation. “Standing waves” are thought to be due to irritation or spasm of the vessel during injection and are relieved by catheter removal or intra-arterial infusion of a vasodilator. This phenomenon should not be confused with FMD.
Atherosclerosis
FMD is usually associated with young individuals without cardiovascular risk factors, making it easier to distinguish from atherosclerotic disease. Atherosclerotic disease is most likely to occur at branch points in the proximal portions of the brachiocephalic arteries and the carotid bifurcation and often show signs of calcification within the plaque or vessel wall. FMD is located in the distal portions of the ICA and vertebral arteries, or in ECA branches, and has the “string of beads” appearance, which is not associated with ASVD.
Inflammatory Arteriopathies
Large artery vasculitides, such as Takayasu’s and giant cell arteritis, may mimic the tubular stenoses noted with FMD. However, these arteriopathies often consist of long, smooth tapering stenoses of the brachiocephalic origins and proximal aspects of the carotid and vertebral artery, which are not typical locations for FMD. Inflammatory markers, such as erythrocyte sedimentation rate and C-reactive protein, are often positive in inflammatory arteriopathies and not in FMD.
Spontaneous Dissection
Although FMD is implicated in spontaneous cerebrovascular dissections, other etiologies include collagen-vascular disorders, such as Marfan’s and Ehlers-Danlos syndromes; atherosclerosis; hyperextension during sports or exercise; and mild blunt force trauma. Evaluation of uninvolved cervicocerebral vessels or the renal arteries may show the typical findings of FMD to make the diagnosis.
Segmental Arterial Mediolysis
This noninflammatory, nonatherosclerotic arteriopathy is caused by lysis of the outer media of the arterial wall, resulting in segmental changes including stenoses, dissections, and aneurysm formation. The imaging characteristics are often indistinguishable from FMD, and pathologic findings may also be similar. However, the clinical profile and location of involved vessels in segmental arterial mediolysis (SAM) is markedly different from FMD. SAM most commonly involves the splanchnic vessels of late middle-aged and elderly individuals who present with abdominal or flank pain, distention, falling hematocrit, and hypovolemic shock. Dissections of peripheral arteries unrelated to the aorta with pseudoaneurysm formation, often multiple, is indicative of SAM.
Imaging Evaluation
The increased detection of cerebrovascular FMD may be associated with the increased awareness of its association in the presence of renal FMD and the availability of high-quality noninvasive imaging that permits evaluation of vascular territories outside of the symptomatic one. Duplex ultrasound, CTA, and magnetic resonance angiography (MRA) are all suitable for cerebrovascular FMD diagnosis, and the latter two have the additional advantage of allowing concurrent parenchymal evaluation. However, catheter angiography remains the diagnostic gold standard, and flow and pressure measurements across stenoses can be performed at the same.
Duplex Ultrasound
This imaging modality is noninvasive, widely available, and relatively inexpensive and gives both anatomic and flow-related information. Velocity shifts indicative of stenosis with associated turbulence of color flow, beading, and tortuosity of the mid-to-distal cervical ICA are evidence of FMD. One limitation of the technique is the lack of specific velocity-based criteria for severity of stenosis that has been angiographically validated. The standards for atherosclerotic stenosis do not apply, and less-skilled vascular labs may misinterpret FMD for atherosclerotic stenosis. Duplex ultrasound can be used as surveillance imaging, with annual studies performed initially, and less frequent testing after stability has been recognized. This approach was given a class IIa recommendation by the 2011 multisocietal guidelines for extracranial carotid and vertebral disease.
CTA and MRA
There are no clinically validated studies in the comparison of CTA or MRA to catheter angiography for the diagnosis of cerebrovascular FMD. However, multidetector CTA is routinely used for the evaluation of extracranial and intracranial disease, including atherosclerosis, FMD, dissection, and cerebral aneurysms, and displays detailed anatomy in multiple planes using maximal intensity and three-dimensional projections. T1-fat saturation sequences are particularly useful in the detection of FMD-associated dissections. MRA has the advantage of not using radiation or iodinated contrast making it a useful screening tool, but its sensitivity and specificity in the detection of FMD are unknown.
Cerebral Angiography
Digital subtraction angiography (DSA) is the “gold standard” in the evaluation of FMD due to its superb spatial resolution and digital magnification capabilities that allows disease detection in the smallest vessels. The three most common angiographic patterns identified are the “string of beads” appearance, smooth tubular stenosis, and smooth tubular dilatation with an associated outpouching. Also, DSA has a higher detection rate of small intracranial aneurysms than does CTA or MRA.
However, the diagnosis of FMD is readily and reliably made with high-quality noninvasive imaging, and angiography is often reserved for symptomatic patients in whom intervention is contemplated, when the diagnosis or severity is in question, or for hemodynamic evaluation or investigation of a source of thrombus.
Treatment of Cerebrovascular FMD
Medical Therapy
The incidental finding of FMD may require nothing more but the knowledge of its presence and surveillance. The decision to treat is dependent upon the nature of the lesion, (for example, stenosis, dissection, and degree of vascular involvement); the presence of symptoms and prior events related to the disease; presence and size of intracranial aneurysms or extracranial pseudoaneurysms; and comorbidities. There are no randomized controlled trials in this patient population to provide guidance.
The 2011 multisocietal statement supported the following medical therapies in patients with FMD:
1. Use of antiplatelet therapy in asymptomatic and symptomatic patients with carotid FMD as long as there was no contraindication for its use (Class IIa evidence)
2. Management of cervical artery dissection with heparin or low molecular weight heparin followed by oral anticoagulation with warfarin for 3 to 6 months and then antiplatelet therapy (Class IIa evidence)
3. Modification of cardiovascular risk factors such as hypertension treatment, smoking cessation, and statin therapy in accordance with published consensus guidelines



