Acute myocardial infarction (AMI) initiates an intense inflammatory response in which interleukin-1 (IL-1) plays a central role. The IL-1 receptor antagonist is a naturally occurring antagonist, and anakinra is the recombinant form used to treat inflammatory diseases. The aim of the present pilot study was to test the safety and effects of IL-1 blockade with anakinra on left ventricular (LV) remodeling after AMI. Ten patients with ST-segment elevation AMI were randomized to either anakinra 100 mg/day subcutaneously for 14 days or placebo in a double-blind fashion. Two cardiac magnetic resonance (CMR) imaging and echocardiographic studies were performed during a 10- to 14-week period. The primary end point was the difference in the interval change in the LV end-systolic volume index (LVESVi) between the 2 groups on CMR imaging. The secondary end points included differences in the interval changes in the LV end-diastolic volume index, and C-reactive protein levels. A +2.0 ml/m 2 median increase (interquartile range +1.0, +11.5) in the LVESVi on CMR imaging was seen in the placebo group and a −3.2 ml/m 2 median decrease (interquartile range −4.5, −1.6) was seen in the anakinra group (p = 0.033). The median difference was 5.2 ml/m 2 . On echocardiography, the median difference in the LVESVi change was 13.4 ml/m 2 (p = 0.006). Similar differences were observed in the LV end-diastolic volume index on CMR imaging (7.6 ml/m 2 , p = 0.033) and echocardiography (9.4 ml/m 2 , p = 0.008). The change in C-reactive protein levels between admission and 72 hours after admission correlated with the change in the LVESVi (R = +0.71, p = 0.022). In conclusion, in the present pilot study of patients with ST-segment elevation AMI, IL-1 blockade with anakinra was safe and favorably affected by LV remodeling. If confirmed in larger trials, IL-1 blockade might represent a novel therapeutic strategy to prevent heart failure after AMI.
Acute myocardial infarction (AMI) initiates an intense inflammatory response characterized by an accumulation of leukocytes in the injured myocardium and the production of cytokines and chemokines, which further promotes adverse cardiac remodeling and heart failure. Interleukin-1 (IL-1) is the prototypic inflammatory cytokine, inducing adhesion molecules and chemokines. IL-1 is also a known myocardial suppressant. In AMI, IL-1 is initially released by the ischemic endothelial cells and cardiomyocytes and, later, by the leukocytes infiltrating the myocardium. Although IL-1 leads to leukocyte recruitment, which contributes to infarct healing, IL-1 also promotes cell death in cardiomyocytes. The naturally occurring IL-1 receptor antagonist binds to the IL-1 receptor and prevents IL-1 activity. We have reported that a recombinant human IL-1 receptor antagonist, anakinra, ameliorated cardiac remodeling after a large anterior wall AMI in the experimental mouse model and improved survival. Moreover, mice with deletion of the IL-1 type I receptor were protected from adverse cardiac remodeling, demonstrating the critical role of IL-1 activity in AMI. We designed and conducted a randomized double-blind pilot trial comparing anakinra and placebo to test the hypothesis that IL-1 blockade would be safe and lead to more favorable left ventricular (LV) remodeling in patients with ST-segment elevation AMI.
Methods
The study design was registered at the ClinicalTrials.gov Website (National Clinical Trial no. 00789724 ). An exemption for investigational new drug use was allowed by the Food and Drug Administration according to the current regulations [Code of Federal Regulations 312.2(b)]. The Virginia Commonwealth University institutional review board approved the study, and all patients provided written consent. Starting on November 16, 2008, consecutive patients presenting to our institution with suspected ST-segment elevation AMI were screened for enrollment ( Figure 1 ). The inclusion criteria were age >18 years, acute (<24 hours) onset of chest pain, new or presumably new ST-segment elevation (>1 mm) in ≥2 anatomically contiguous leads, and planned or completed angiography for urgent percutaneous coronary intervention. The exclusion criteria were a lack of informed consent; unsuccessful percutaneous revascularization or the need for urgent surgical revascularization; hemodynamic instability requiring the use of an intra-aortic balloon pump; dopamine, dobutamine, norepinephrine, or epinephrine infusions; pre-existing congestive heart failure stage C/D, New York Heart Association class IV; severe LV dysfunction (LV ejection fraction <20%) or severe aortic or mitral valve disease; contraindications to magnetic resonance imaging; pregnancy; chronic autoinflammatory or autoimmune disease; severe asthma; severe coagulopathy (international normalized ratio >2.0 or platelet count <50,000/mm 3 ); or recent (<14 days) use of anti-inflammatory drugs (nonsteroidal anti-inflammatory drugs excluded).
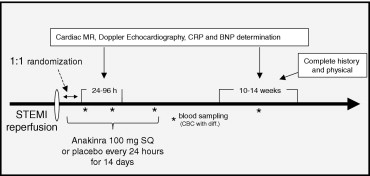
Randomization was performed by the investigational pharmacist using a dedicated randomization algorithm (available on-line at www.metcardio.org ). The investigator in charge of randomization was not involved in patient care, data gathering, or data analysis. Anakinra (Amgen, Thousand Oaks, California) was purchased from the investigational pharmacy. For each patient, the pharmacist prepared a set of 14 syringes containing 100 mg of anakinra in 0.67 ml or matching syringes containing sodium chloride 0.9% placebo that were indistinguishable from the treatment syringes. Treatment consisted of 14 daily subcutaneous injections. The patients were monitored in the intensive care unit for >48 hours and were assessed daily for adverse treatment effects. A complete blood cell count was obtained at admission and daily for 4 days. A group of consultants (see Appendix ) was available to the investigators to provide advice regarding potential adverse effects.
A cardiac magnetic resonance (CMR) study and Doppler echocardiography were performed 24 to 96 hours after admission and 10 to 14 weeks later. High-sensitivity C-reactive protein and brain-type natriuretic peptide were measured at 72 hours, 14 days, and 10 to 14 weeks. Serial cardiac markers were obtained, as clinically indicated. Clinical follow-up was performed in-hospital until discharge and then at the Cardiology Research Clinic at day 14 and 10 to 14 weeks later.
The primary end point was the difference in interval changes in the LV end-systolic volume index (LVESVi) assessed by CMR imaging from baseline to follow-up, comparing the anakinra- and placebo-treated patients. The secondary end points included the difference in interval changes in the LVESVi, as assessed by echocardiography, and the interval changes in the LV end-diastolic volume index, LV ejection fraction, LV mass, infarct size, wall motion score index, and estimated cardiac index, as assessed by CMR or echocardiography. The CMR studies were obtained in the Virginia Commonwealth University Cardiac Magnetic Resonance Imaging suite using a Siemens Avanto 1.5 Tesla magnet (Munich, Germany). After the initial localizer sequences in the transverse, frontal, and sagittal planes, contiguous 6-mm steady-state free precession cine images with 2-mm gaps were obtained from the mitral valve ring though the cardiac apex. The LV end-systolic volume, LV end-diastolic volume, and LV ejection fraction were computed by tracing the endocardial and epicardial LV contours in systole and diastole using this stack of 10 to 12 short-axis slices. Gadolinium was then administered through a peripheral intravenous line. To visualize the areas of infarction and no reflow, late gadolinium enhancement imaging was performed, beginning 10 minutes after contrast administration using a standard segmented inversion recovery gradient echo sequence in the short-axis plane at locations that spatially matched the cine acquisitions. The infarct size was calculated using a 17-segment model with the degree of transmurality graded by quartiles (0 to 4 scale). Partial volume effects were evaluated using a grading scale (0 to 4) for the visual intensity of enhancement. A value of 4 was assigned to the brightest region of enhancement and 0 to remote myocardium. Echocardiographic studies were obtained using an HP Phillips Sonos 5500 (Andover, Massachusetts) or IE33 apparatus with dual harmonic imaging and short- and long-axis views, as recommended by the American Society of Echocardiography. The LV end-systolic volume, LV end-diastolic volume, and LV ejection fraction were computed in the apical 4- and 2-chambers views over multiple cycles, using the modified Simpson formula. The wall motion score index was calculated as the ratio of the sum of the score from each of 16 segments using standard scoring recommendations divided by the total number of segments. Transmitral flow and LV outflow tract flow Doppler spectra and the annular tissue Doppler spectra were obtained from the apical window, and the cardiac index was calculated. All measurements and calculations were performed at the end of the study jointly by 2 investigators, who were unaware of the patient treatment, and decisions were made in consensus. The white blood cell count, brain-type natriuretic peptide, troponin I, creatine kinase-MB, creatinine, and other routine tests and measurements were performed in the Virginia Commonwealth University Health System Pathology Laboratories. The determination of the high-sensitivity C-reactive protein levels was performed by LabCorp (Burlington, North Carolina) using high-sensitivity rate nephelometry.
Given the limited funding for the present pilot study, we chose a sample size of 10 patients to provide >50% power to detect an estimated average intergroup difference in LVESVi ≥4 ml/m 2 , with an anticipated SD of ≤6 ml/m 2 and an α of 0.05. Statistical analyses were conducted in a blinded fashion. At study completion, one investigator was made aware of the allocation to group 1 or 2, but without knowledge of the treatment received. Once the analyses were completed, the randomization code was opened and made available to all the investigators. The values are reported as the median and interquartile range for potential deviation from the gaussian distribution. The differences between the 2 groups were computed using the Wilcoxon test for continuous variables or Fisher’s exact test for discrete variables. The differences in interval changes between the 2 groups were compared using random-effect analysis of variance for repeated measures to analyze the effects of time and group allocation. The Spearman correlation test was used to evaluate the correlation between the 2 variables. Unadjusted p values are reported throughout, with statistical significance set at the 2-tailed 0.05 level. The analyses were completed using the Statistical Package for Social Sciences, version 11.0.1, software (SPSS, Chicago, Illinois).
Results
Enrollment started in November 2008. During the first 4 months, 33 patients were admitted with ST-segment elevation AMI and screened, and 10 patients were enrolled ( Figure 2 ). One patient withdrew consent to the study on day 2 before all assessments had been completed and was excluded. The institutional review board then approved enrollment of an additional patient, who was enrolled in May 2009. The demographic and clinical characteristics of the patients are summarized in Table 1 . No significant differences in age, gender, ethnicity, risk factors, or clinical characteristics were present between the 2 groups. The interval from admission to the CMR study was not signficantly different statistically between the 2 groups (47 hours, range 41 to 85, vs 54 hours, range 50 to 77) for anakinra and placebo, respectively (p = 0.8). The infarct size at late gadolinium enhancement on CMR imaging was 15.4% in the anakinra group and 14.3% in the placebo group (p = 0.39), and the size of no-reflow was small in both groups (0% and 1.3% for the anakinra and placebo groups, respectively; p = 0.44). All patients were discharged with aspirin 325 mg/day, clopidogrel 75 mg/day, metoprolol 25 to 150 mg/day (or equivalent), fosinopril 2.5 to 40 mg/day (or equivalent), atorvastatin 10 to 80 mg/day (or equivalent), with no significant differences in the dosages between the 2 groups.
| Pt. No. | Age (yrs) | Gender | Ethnicity | Hypertension | Diabetes | Time From Chest Pain to PCI (min) | Time From Chest Pain to Drug (min) | Thrombolysis (Before PCI) | CAD (culprit) | CAD (other) | TIMI Flow (Initial) | TIMI Flow (Final) | Coronary Stenting | Infarct Size (%) | Adverse Clinical Events | LVESVi (Admission) | LVESVi (Follow-Up) | Change in LVESVi (ml/m 2 ) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | ||||||||||||||||||
| 1 | 60 | M | W | Y | 5.0 | 75 | 289 | N | RCA | N | 1 | 3 | Y | N | Acute coronary syndrome; Femoral artery pseudoaneurysm | 12.0 | 23.5 | +11.5 |
| 3 | 28 | M | W | N | 14.3 | 664 | 1020 | Y | RCA | N | 2 | 3 | Y | N | None | 21.0 | 23.0 | +2.0 |
| 5 | 65 | M | B | Y | 25.8 | 264 | 515 | Y | LAD | RCA | 0 | 3 | Y | Y | Heart failure | 29.0 | 28.5 | −0.5 |
| 6 | 53 | M | W | N | 7.1 | 115 | 480 | N | RCA | LCX | 1 | 3 | Y | N | Unstable angina | 41.5 | 42.5 | +1.0 |
| 8 | 45 | M | B | Y | 19.4 | 139 | 390 | N | LCX | RCA | 0 | 3 | Y | N | Heart failure, atypical chest pain | 41.5 | 61.5 | +20.0 |
| Anakinra | ||||||||||||||||||
| 2 | 59 | M | W | Y | 12.1 | 64 | 453 | N | LCX | LAD RCA | 0 | 3 | Y | Y | None | 42.5 | 34.5 | −8.0 |
| 7 | 35 | F | W | Y | 27.0 | 415 | 732 | N | LCX | N | 1 | 3 | N | Y | None | 48.5 | 47.0 | −1.5 |
| 9 | 59 | M | W | Y | 13.7 | 192 | 470 | N | RCA | LAD | 1 | 3 | Y | N | None | 24.5 | 21.5 | −3.0 |
| 10 | 40 | M | W | Y | 25.8 | 267 | 472 | Y | RCA | N | 3 | 3 | Y | N | Injection site pain | 52.5 | 48.0 | −4.5 |
| 11 | 34 | F | W | Y | 15.4 | 140 | 583 | N | LAD | LCX | 0 | 3 | Y | N | Acute coronary syndrome, injection site pain | 31.0 | 29.5 | −1.5 |
All patients were alive at the 10- to 14-week follow-up visit. Adverse clinical events are listed in Table 1 . Two patients (both in the anakinra group) reported injection site pain; one of whom required analgesics and topical steroids. No patients experienced systemic side effects. At follow-up, a +2.0 ml/m 2 median increase (interquartile range +1.0 to +11.5) was seen in the LVESVi in the placebo group and a −3.2 ml/m 2 median decrease (interquartile range −4.5 to −1.6) in the anakinra group (p = 0.033), with a median difference of 5.2 ml/m 2 in the LVESVi change between the 2 groups (primary end point, Figure 3 ; Table 1 and Table 2 ). The LVESVi measurements by echocardiography correlated highly with the measurements by CMR imaging (R = +0.91, p <0.001), with a relative underestimation of volumes with echocardiography. By echocardiography, anakinra-treated patients had a −2.7 ml/m 2 (interquartile range −4.5 to −1.8) decrease in LVESVi compared to a + 10.7 ml/m 2 (interquartile range +4.2 to +11.2) increase in the placebo group (p = 0.006), leading to a median difference of 13.4 ml/m 2 between the 2 groups ( Figure 3 and Table 1 ). Similar trends were noted in the LV end-diastolic volume index by CMR imaging (median difference 7.6 ml/m 2 , p = 0.033) and echocardiography (median difference 9.4 ml/m 2 , p = 0.008; Figure 3 and Table 1 ). The LV ejection fraction remained unchanged over time by CMR (median change 0% in both groups). In contrast, by echocardiography, a trend was seen toward a mild LV ejection fraction reduction in the placebo group (−3%, interquartile range −3 to −10), with no change in the anakinra group (0%, interquartile range 0 to +6, p = 0.070). No significant changes in LV mass were noted in either group. The infarct size at late gadolinium enhancement was reduced in both groups by approximately 20%, without any difference between the 2 groups. However, a trend was seen toward a more favorable change in the wall motion score index in the anakinra group (−0.25, interquartile range −0.27 to −0.06) compared to the placebo group (0, interquartile range −0.06 to +0.06, p = 0.068). No significant differences were seen in the changes in transmitral Doppler spectra and/or tissue Doppler spectra between the 2 groups. A significant difference in the changes in the cardiac index was noted, with a −0.5 L/min/m 2 (interquartile range −0.4 to −0.7) reduction in the placebo-treated group and a −0.1 L/min/m 2 (interquartile range 0 to −0.1) reduction in the anakinra-treated group (p = 0.029). The blood pressure values were not significantly different statistically between the 2 groups and were unaffected by treatment (data not shown).




