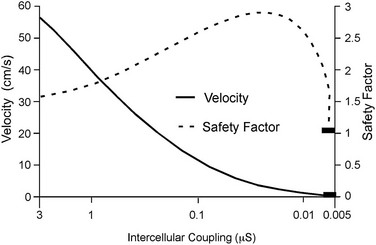
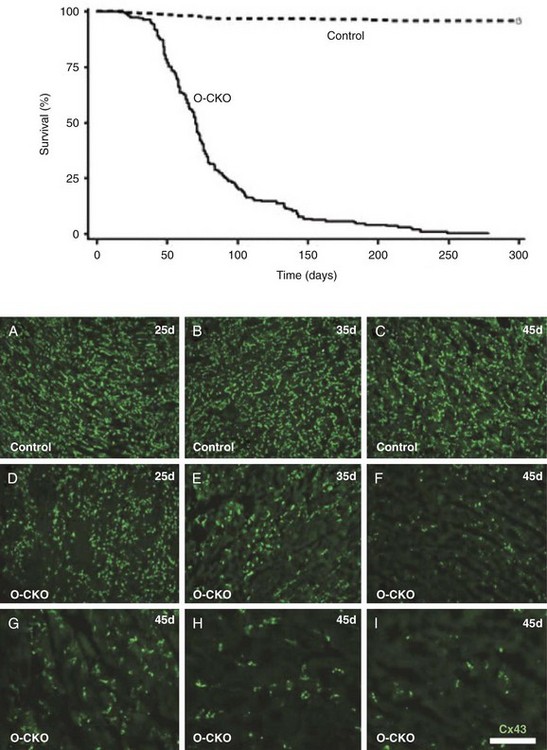
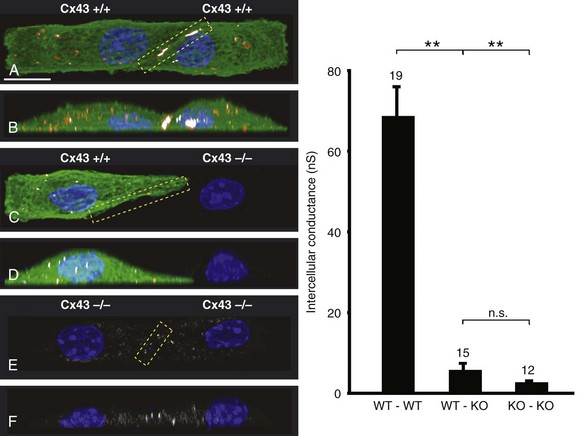
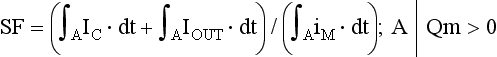27 Cardiac Intercellular Communication by Gap Junctions Myocardial Connexins Cx43, Cx40, and Cx45 Role of Gap Junctions in Electrical Impulse Transfer and Propagation Effect of Heterogeneous Expression of Connexins on Propagation Interaction of Cell-to-Cell Coupling, Tissue Architecture, and Ion Currents Ephaptic Impulse Transmission: Potential Alternative Mechanism of Electrical Impulse Transfer Remodeling of Gap Junctions: Only a Part of Remodeling of the Whole Intercalated Disc? The existence of low-resistance pathways between cardiac cells was postulated from experimental assessment of electronic interactions (cable analysis) between cardiac Purkinje fibers before the existence of gap junction channels was known.1 During the subsequent decades ion channels between cardiac cells were identified, formed from two connexon hemichannels, each being composed of six connexin molecules. The main connexins of the myocardium—Cx43, Cx40, and Cx45—have been defined in terms of genetic code, amino acid sequence, and molecular function. The introduction of the whole-cell dual voltage clamp technique made it possible to measure directly the intercellular electric conductance and single channel conductance between a pair of cells obtained from enzymatic disaggregation of cardiac tissue or after neoformation in cultured cells. Later, transfection techniques made it possible to study the biophysical properties of specific cardiac connexins in heterologous expression systems and to define the properties of pure or mixed connexons and connexins (heteromeric vs. homomeric connexons and homologous vs. heterologous connexin channels) in terms of biophysical and diffusive properties.2–5 Similar to ion channels, the electrical behavior of gap junctions channels is sensitive to the electrical field across the channel and to variations of the intercellular milieu (Ca2+, pH, Mg2+, ATP).6 Gap junction channels have a high turnover rate with a half-time of only a few hours.7 Trafficking of connexins to the membrane along microtubular highways requires the presence of proteins specifically linking the microtubular ends to the scaffold of mechanical junctions.8 The hemichannels (connexons) are transported by the microtubules to the periphery of the gap junction plaques, shift to the center of the plaques in a short time, and are continuously internalized from the plaque center along microfilaments.7 Proteins, such as ZO-1 and CAR, are taking part in these processes and are involved in interactions with other proteins of the intercellular junction and plaque size control.9 The total of these behaviors indicates that gap junctions represent a highly dynamic, regulated, and interactive system and explains why changes in composition and function can contribute importantly to disease phenotypes. Cx43, Cx40, and Cx45 are the three major connexins of the myocardium.10–12 A fourth connexin, Cx32.1, has been described in murine nodal tissues.13 Cx43 is the dominating connexin in ventricular myocardium, Cx40 dominates in the ventricular conduction system, and Cx45 is present in the atrioventricular and sinoatrial nodes and in lower amounts in atrial and ventricular myocardium. Atrial tissue shows a wide spectrum of electrical propagation velocities (0.2 to 1.6 m/s).14 This raises the question to what extent Cx43 and Cx40, which are both abundantly expressed and colocalize in intercalated discs (the ratio of Cx40:Cx43 expression is larger in the right than the left atrium15), contribute to this variability. In human atria, an important factor in determining propagation is the ratio of Cx40:Cx43 expression, with Cx40 dominance decreasing and a Cx43 dominance increasing local velocity.16 A similar behavior was observed in vitro in atrial strands cultured from myocytes with genetic Cx43 and Cx40 ablation.17 In contrast, measurements of macroscopic excitation in murine atria with Cx40 ablation in vivo yielded slowed or unchanged propagation.17 It cannot be excluded that macroscopic measurements in whole atria with genetic Cx40 ablation are affected by malformations.18 Colocalization of Cx43 and Cx40 raises the question of whether these connexins form separate gap junctions or whether mixed Cx40/Cx43 gap junction channels exist in the real atrial myocardium. This question is especially interesting regarding their effect on propagation, because mixed Cx40/Cx3 channels could have an electrical conductance different from pure homomeric or homotypic Cx43 or Cx40 channels.2,19 In heterologous expression systems, controversial results about the function of mixed Cx40/Cx3 channels have been reported.2,19–20 Cx45 is a dominating connexin in early stages of cardiac development and in the atrioventricular and sinoatrial nodes. In atrial and ventricular tissue from human, rat, and murine hearts, Cx45 has been detected in small amounts,15,17,21–22 where it is likely to form mixed channels with Cx43 and/or Cx40. Heteromeric connexins have been described in heterologous expression systems, if Cx45 was coexpressed with Cx43 or Cx40.2 Germline ablation of Cx43 in murine ventricular pairs produces a greater than 90% reduction in cell-to-cell coupling with persistence of Cx45 expression.22 This finding indicates that Cx45 contributes little to electrical coupling; however, a regulatory role has been attributed to Cx45 in cardiac failure, in which Cx45 is upregulated together with a decrease in Cx43 and a reduction in gap junction size.23 This could indicate that Cx45 is involved in regulation of gap junction size, as also suggested from coexpression of Cx43 with Cx45 in a rat liver epithelial cell line.24 Recently, it has been shown that genetic deletion of the Coxsackie-adenovirus receptor (CAR) is associated with marked reduction or deletion of Cx45. Conditional ablation of CAR increased cell-to-cell dye diffusion between ventricular cells, further suggesting that Cx45 is a significant modulator of ventricular cell-to-cell communication.25 The role of gap junction channels in cardiac propagation as one of the key elements is well acknowledged. Early experimental and theoretical studies examined the effect of a decrease in gap junction conductance on propagation velocity in a general way by implicating or inducing a uniform change of intercellular electrical conductance in the tissue.26 The effect of a uniform decrease in intercellular electrical conductance is best understood by considering the principle of conduction safety. The formalism describing conduction safety (SF) states that27: In simple terms, the numerator of this equation equals the electrical charge produced by a given cell in the propagating wavefront, and it consists of the sum of electrical charges needed to produce the action potential (capacitive component, ∫AIC ⋅ dt) and the charge flowing to excite the cells downstream (axial component, ∫AIout ⋅ dt). The denominator corresponds to the electrical charge (∫AIm ⋅ dt) exciting this same cell, which in turn is produced by the excited cells upstream of the propagating front. From this formalism, it follows that propagation becomes decremental and eventually gets blocked, when SF decreases less than 1, or when more electrical charge is needed to excite a given cell than this same cell can produce by its machinery of excitation.27 The theoretical dependence of propagation velocity on cell-to-cell coupling and the associated change in SF is illustrated in Figure 27-1. Several distinct features specifically related to cellular uncoupling can be derived from this figure. First, changes in cell-to-cell coupling close to the normal level of coupling produce relatively small changes in propagation velocity. Second, cell-to-cell uncoupling can produce extremely slow propagation (on the order of 1 cm/s) if the conductance between cells is reduced more than 100-fold. This behavior also predicts preservation of propagation, albeit slow, even at extreme levels of uncoupling. This prediction made from theoretical studies has been verified in experimental work using engineered strands of neonatal rat ventricular myocytes either exposed to an uncoupling agent or using cells with germline ablation of connexin43 (Cx43).22,28 The explanation for this behavior, which is typical for cell-to-cell uncoupling and contrasts to the behavior during block of depolarizing ion currents (INa, IL,Ca),27–28 is given by the biphasic behavior of the margin of safety of propagation (see Figure 27-1). The biphasic time course of SF is determined by two processes with opposing effects. First, the axial currents flowing into a given cell from excited tissue upstream decreases because of an increase in the resistance between the cells. This decrease (so-called source effect) causes the membrane capacitance of a given cell to charge more slowly and to reach the excitation threshold later than normal, and therefore, propagation to slow. Second, the excitatory electrical charge flowing downstream, because of the increased intercellular resistance between the downstream cells, is distributed over fewer cells and is therefore conserved at the site of excitation within the wavefront (decrease of so-called sink effect). In other words, cell-to-cell uncoupling protects the excitatory current from downstream dissipation and therefore makes propagation safer (increase of SF). Only at extreme levels of uncoupling does failure occur and propagation eventually gets blocked. The biophysical principle of source-sink interaction is not only valid for states of cell-to-cell uncoupling; it is a general rule independent of scale and holds also for propagation in the presence of discontinuities in tissue structure (e.g., infarct scars26). Importantly, slow propagation is only preserved if sites of increased coupling resistance are closely spaced and produce an effective decrease in the electrotonic sink. If this effect is absent, such as at the transition between tissue of decreased to normal coupling, propagation block is observed.29 This likely explains some observations made in experimental models of heterogeneous connexin expression,30 which is discussed as follows. Figure 27-1 Dependence of safety and velocity of propagation on intercellular coupling. Note logarithmic scale on abscissa. (From Shaw RM, Rudy Y: Ionic mechanisms of propagation in cardiac tissue. Roles of the sodium and l-type calcium currents during reduced excitability and decreased gap junction coupling. Circ Res 81:727–741, 1997.) Heterogeneous expression of connexins, leading to heterogeneous propagation and arrhythmogenesis, has been implicated in the genesis of atrial fibrillation and ventricular arrhythmias.31 However, in only two studies has a direct association between the incidence of ventricular arrhythmias in heart failure patients with heterogeneous connexin expression been demonstrated.32–33 Interestingly, Cx43 immunofluorescence measured in specimens from such patients with ventricular arrhythmias showed a heterogenous pattern with scattered absence of Cx43 signals. Similarly, in a mouse model of conditional cardiac Cx43 ablation, it has been shown that the occurrence of ventricular arrhythmias and sudden cardiac death is associated with a marked heterogeneity in connexin expression and a decrease in macroscopic propagation velocity less than 50% of normal (Figure 27-2).34 Recent work using cell engineering techniques made an attempt to quantitatively study electrical cell-to-cell coupling and propagation in tissue with reproducible degrees of connexin heterogeneity.35 Heterogeneity was produced in engineered cell pairs and strands from defined mixtures of wild type (WT) neonatal ventricular myocytes expressing a green fluorescent protein (GFP) tag (WT-GFP) and Cx43–/– ventricular myocytes (Figure 27-3). Immunofluorescence analysis showed coexistence of Cx43 and Cx45 at the cell interface in the WT/WT pairs. In contrast, no Cx45 or Cx43 signals were observed in mixed WT/CX43–/– pairs, and no Cx45 signals in pairs consisting of Cx43–/– cells. In the same pairs, intercellular electrical conductance (gj) decreased markedly to less than 90% of WT in the mixed pairs (WT/Cx43–/–) and Cx43–/–/Cx43–/– pairs. However, all three cell interfaces showed consistent electrical coupling. For the mixed cell pairs, the dependence of gj on the transjunctional voltage, Vj, was typical of pairs with Cx45 expression in one cell and WT Cx43/Cx45 expression in the other.36–37 The absence of a Cx43 signal in the presence of reduced persistent electrical coupling indicated that the electrical conductance is a more sensitive indicator of the level of cell-to-cell coupling than immunofluorescence, and it confirmed recent measurements correlating electrical conductance to gap junction size as assessed by immunofluorescence.38 The experimental model of engineered cell strands consisting of controlled mixtures of WT-GFP and Cx43–/– cells was used to measure the effect of Cx43 inhomogeneity on propagation velocity (Figure 27-4).35 The change in macroscopic propagation velocity along engineered strands revealed a relatively preserved velocity in mixtures containing ≥50% WT-GFP cells, and a marked decrease in cell mixtures with ≤50% WT-GFP cells. The explanation for preserved propagation at a relatively fast level, even in the presence of 50% of cells devoid of Cx43 expression, is illustrated in Figure 27-5, which depicts the pattern of cell-to-cell propagation recorded at high spatial and temporal resolution. Both the sequence of activation times and the shapes of the action potential upstrokes reveal a mixed type of propagation, with fast continuous conduction26 meandering through the clusters with normal Cx43 expression, and delayed activation of Cx43–/– cells showing a prolonged foot potential before the rapid action potential upstroke, characteristic of discontinuous conduction.26 Although local propagation delays at cell borders can be as long as several milliseconds, they are too short to produce microentry in markedly uncoupled tissue.22,28,35 Restriction of Cx43 ablation to small cell clusters and eventual excitation of all cells probably explains why optical recordings of macroscopic velocities in the mouse model with conditional Cx43 ablation show relatively smooth isochronal activation.39 Furthermore, the observation that Cx43 immunosignals are absent from the interface between Cx43-expressing and nonexpressing cells suggests that the assessment of the degree of Cx43 ablation (proportion of Cx43–/– cell) is overestimated if calculated from the percent loss of Cx43–/– immunosignal. Figure 27-2 Mouse model of heterogeneous Cx43 expression with delayed conditional Cx43 knock out (O-CKO). Top, Kaplan-Meier survival curve showing onset of sudden death at approximately 30–40 days. Bottom, Cx43 immunofluorescence signals in ventricle between 25 and 45 days after birth in control (A–C), and O-CKO mice (D–F). Inhomogeneity of Cx43 expression by comparison of three different regions of interests (G–I). (From Danik SB, Liu F, Zhang J, et al: Modulation of cardiac gap junction expression and arrhythmic susceptibility. Circ Res 95:1035–1041, 2004.) Figure 27-3 Cell-to-cell coupling at the interface between GPF-tagged Cx43+/+ (wild type [WT]) and Cx43–/– (knockout [KO]) neonatal rat ventricular myocytes. Left, Top and side view of WT/WT cell pair (A, B); WT/KO cell pair (C, D) and KO/KO cell pair (E, F). Cx43 immunofluorescence is in orange; Cx45 immunofluorescence is in white. Note superimposition of Cx43 and Cx45 immunofluorescence at the cell-to-cell interface in A and B and the absence of immunofluorescence in C–F. Right, Intercellular conductance between the pairs is illustrated on the left
Intercellular Communication and Impulse Propagation
Cardiac Intercellular Communication by Gap Junctions
Myocardial Connexins Cx43, Cx40, and Cx45
Role of Gap Junctions in Electrical Impulse Transfer and Propagation
Effect of Heterogeneous Expression of Connexins on Propagation
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Intercellular Communication and Impulse Propagation