New-onset rhythm conduction disorders are frequent after transcatheter aortic valve implantation (TAVI). Multidetector row computed tomography may shed light on the pathophysiology of rhythm conduction disorders in patients who undergo TAVI with the Edwards SAPIEN valve. A total of 94 patients (mean age 81 ± 7 years, 48% men) treated with TAVI with the Edwards SAPIEN valve who underwent pre- and post-TAVI multidetector row computed tomography were included. Patients with preexisting right bundle branch block or left bundle branch block (LBBB) and permanent pacemakers were excluded. Pacemaker implantation or new-onset LBBB at 1-month follow-up was the combined end point. Overall, 1 pacemaker was implanted, and 14 cases of new-onset LBBB were recorded. Among several clinical and multi-detector row computed tomographic variables, overexpansion of the transcatheter valve >15% of native annular area (odds ratio 5.277, 95% confidence interval 1.398 to 19.919, p = 0.014) and depth of frame into the left ventricular outflow tract (odds ratio 1.401, 95% confidence interval 1.066 to 1.770, p = 0.010) were independently related to the need for a pacemaker or new-onset LBBB. In conclusion, overexpansion of the transcatheter prosthesis by >15% of native aortic annular area and implantation depth of the frame into the left ventricular outflow tract were independently associated with the need for a pacemaker or new-onset LBBB in patients who underwent TAVI with the Edwards SAPIEN valve.
Highlights
- •
New-onset conduction disorders 1 month after TAVI were evaluated.
- •
Pre- and postoperative computed tomography was available in all included patients.
- •
Frame deployment in the aortic annulus and LVOT was defined.
- •
Overall, 1% of patients required new pacemakers, and 15% had new-onset LBBB.
- •
Implantation depth and overexpansion >15% of the aortic annulus were related to new conduction disorders.
New-onset persistent left bundle branch block (LBBB) and need for permanent pacemaker implantation after transcatheter aortic valve implantation (TAVI) have been described in 10% to 19% and 2.5% to 11.5% of patients receiving Edwards SAPIEN prostheses (Edwards Lifesciences, Irvine, California), respectively. Baseline QRS complex duration and deep implantation of the prosthesis into the left ventricular outflow tract (LVOT) have been consistently associated with these complications after TAVI. The higher spatial resolution of multi-detector row computed tomography (MDCT) allows a more accurate evaluation of the spatial relations of an implanted prosthesis into the aortic root and may shed light into the pathophysiology of the development of new-onset LBBB and need for permanent pacemaker implantation in patients who undergo TAVI. In the present study, we evaluated the multidetector computed tomographic correlations of new-onset LBBB and need for a pacemaker in patients treated with TAVI.
Methods
The study included 94 patients who underwent TAVI with Edwards SAPIEN valves (SAPIEN or SAPIEN XT) at Leiden University Medical Center (Leiden, The Netherlands). Information about inclusion criteria and procedural details have been previously described in detail. For this analysis, patients with preexisting right bundle branch block (RBBB) or LBBB and those with permanent pacemakers were excluded. Also, patients with bioprosthetic aortic valves and valve-in-valve implantation as a bailout procedure were excluded. Unless contraindicated, patients referred for TAVI were evaluated with preprocedural MDCT for accurate measurement of the native aortic annulus and valve sizing. One month after TAVI, repeat MDCT was performed to define the deployment of the prosthesis in the LVOT and aortic root. After successful TAVI procedures, patients were followed at the outpatient clinic with scheduled visits at 1, 3, and 12 months. Clinical evaluation, surface electrocardiography, and echocardiography were routinely performed. All clinical information on demographics, electrocardiography (ECG), and imaging techniques was digitally stored in the departmental database (EPD Vision version 8.3.3.6; Leiden, The Netherlands) and were retrospectively analyzed. For this study, baseline clinical data in combination with pre- and post-TAVI MDCT parameters were related to the combined end point (need for pacemaker implantation or new-onset LBBB at 1-month follow-up). For this retrospective analysis of clinically acquired data, the institutional review board waived the need for patient written informed consent.
All electrocardiograms were retrospectively reviewed at 3 time points: at baseline (before TAVI), during hospitalization, and at 1-month follow-up. The presence of RBBB and LBBB was diagnosed according to criteria recommended by the World Health Organization and International Society and Federation for Cardiology Task Force. Durations of the PR interval and QRS complex were automatically calculated with dedicated software (Mega Care ECG Management System; Dräger, Lubeck, Germany).
A 64- or a 320-detector-row computed tomographic scanner was used for preoperative and postoperative scanning of the patients. When the Aquilion 64 system (Toshiba Medical Systems, Otawara, Japan) was used, data were acquired with collimation of 64 × 0.5 mm and a gantry rotation time of 400 ms (tube current was 300 to 400 mA, and voltage was 120 kV or 135 kV), and when the Aquilion ONE system was used, data were acquired with collimation of 320 × 0.5 mm (gantry rotation time 350 ms, tube current and voltage set at 400 to 580 mA and 100, 120, or 135 kV, respectively, according to patient’s body mass index). According to the acquisition protocol, patients with heart rates ≥70 beats/min received β blockers unless contraindicated. A volume of 80 to 90 ml of nonionic contrast (Iomeron 400; Bracco, Milan, Italy) was used according to the patient’s body surface area. Scans were acquired in midinspiratory breath hold, and data was digitally stored.
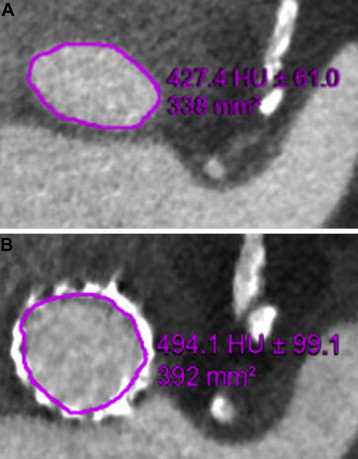
The Agatston scores of the aortic valve annulus and the LVOT were calculated using dedicated postprocessing workstations (Vitrea 2; Vital Images, Minneapolis, Minnesota). Calcification of the LVOT (subannular landing zone) was semiquantitatively graded (1 = none, 2 = mild, 3 = moderate, 4 = severe). Accurate measurements of the aortic annulus and root dimensions were performed. In diastole (75% of the RR interval) coronal, sagittal, and transverse orthogonal planes of the aortic root were reconstructed. The aortic annulus was then defined with alignment of the transverse plane at the level of the hinge points of the aortic cusps, as previously described. Minimal and maximal diameter of the native annulus were measured, and annular area was planimetered ( Figure 1 ). On post-TAVI MDCT, the expansion of the aortic valve was defined at the same level of the aortic annulus. Using the inner margins of the expanded frame as a reference, the maximum and minimum diameters were measured and the effective area of expansion was planimetered ( Figure 1 ). To define the depth of implantation, the distance between the rim of the frame in the LVOT and the native aortic annulus was measured ( Figure 2 ). The ratio of (effective planimetered prosthesis area − MDCT-derived aortic annular area) to MDCT-derived aortic annular area was calculated from pre- and post-TAVI multidetector computed tomographic measurements as a parameter of prosthesis expansion. Prosthesis overexpansion was considered significant when the area of the expanded frame was >15% larger that the nominal aortic valve area.

SPSS version 20 (SPSS, Inc., Chicago, Illinois) was used for all statistical analyses. Continuous variables were considered normally or not normally distributed on the basis of visual inspection of the histograms and are presented as mean ± SD or medians and interquartile ranges, respectively. Categorical variables are presented as numbers and frequencies. Patients were categorized according to the need for new pacemaker implantation or the induction of new-onset LBBB (patients with the combined end point vs patients free of the combined end point). Continuous variables were compared by using unpaired Student’s t tests if normally distributed and Mann-Whitney tests otherwise. Categorical variables were compared by using chi-square tests or Fisher’s exact tests, as indicated. Binary logistic regression analysis was used for the evaluation of the occurrence of the combined end point at 1-month follow-up, and the estimated odds ratios and 95% confidence intervals were calculated. Variables with p values <0.01 in the univariate model were included in the multivariate analysis. Two-sided p values <0.05 were considered statistically significant.
Results
From an initial cohort of 161 patients in whom pre- and postprocedural MDCT was available, 21 patients with RBBB, 24 patients with LBBB, and 22 patients with permanent pacemakers at baseline were excluded. Baseline clinical and echocardiographic characteristics of the remaining 94 patients (mean age 81 ± 7 year, 48% men) are listed in Table 1 . In 9 patients (10%), reballooning of the implanted prosthesis was performed to minimize paravalvular aortic regurgitation.
| Variable | n = 94 |
|---|---|
| Age (years) | 81 ± 7 |
| Male | 45 (48%) |
| Body surface area (m 2 ) | 1.71 ± 0.34 |
| Creatinine (μmol/L) | 87 (70–101) |
| Hypertension | 40 (43%) |
| Diabetes mellitus | 28 (30%) |
| Smoker | 22 (23%) |
| Coronary artery disease | 65 (70%) |
| Coronary bypass | 24 (26%) |
| Medications | |
| Beta-blockers | 53 (56%) |
| Diuretics | 57 (61%) |
| Statins | 57 (61%) |
| Ca-antagonists | 29 (31%) |
| Logistic Euroscore (%) | 20.0 ± 11.7 |
| Transfemoral TAVI | 38 (40%) |
| Transapical TAVI | 56 (60%) |
| Balloon post dilatation | 9 (10%) |
| Edwards SAPIEN valve | |
| 23 mm | 28 (30%) |
| 26 mm | 62 (66%) |
| 29 mm | 4 (4%) |
| Aortic valve area (cm/m 2 ) | 0.72 ± 0.19 |
| Intra-ventricular septum thickness (cm) | 1.4 ± 0.2 |
| Mean transaortic gradient (mmHg) | 43 ± 17 |
| Maximum transaortic gradient (mmHg) | 70 ± 25 |
| Left ventricular ejection fraction (%) | 51 ± 12 |
| AGATSTON score of aortic valve and LVOT (units) | 2927 ± 1643 |
| LVOT ‘landing zone’ calcification (grade 1–4) | 2 (2–3) |
| Preoperative ECG | |
| Left axis deviation | 72 ± 12 |
| Atrial Fibrillation | 14 (15%) |
| PR (ms) | 179 ± 24 |
| QRS duration (ms) | 98 ± 10 |
| Left axis deviation | 7 (7%) |
Compared with baseline ECG, there was a significant increase in QRS duration on predischarge ECG (from 98 ± 10 to 112 ± 22 ms, p <0.001) but not in PR interval (from 179 ± 29 to 180 ± 41 ms, p = 0.878). At this time point, new-onset left axis on ECG was observed in 7 patients (7%), and 11 patients (11%) developed new-onset RBBB. New-onset LBBB developed in 15 patients (16%). Moreover, 1 patient (1%) underwent dual-chamber pacemaker implantation 5 days after TAVI because of symptomatic intermittent grade 3 atrioventricular block and LBBB. The implantation depth of the frame in this patient was 6.4 mm ( Figure 2 ), and the frame was overexpanded by >15% of the native aortic annulus.
Compared with predischarge ECG, there were no significant changes in QRS complex or PR interval duration at 1-month follow-up (from 112 ± 22 to 111 ± 23 ms, p = 0.461 and from 180 ± 41 to 179 ± 36 ms, p = 0.781, respectively). On repeat surface ECG, there were 7 patients (7%) with left-axis deviation, 10 patients (10%) with RBBB, and 14 patients (14%) with LBBB. New-onset in-hospital RBBB and new-onset in-hospital LBBB resolved in 1 and 2 patients at 1-month follow-up, respectively. Moreover, 1 patient developed new-onset LBBB at 1-month follow-up. Consequently, the combined end point of the study at 1-month follow-up was met in 15 patients (16%). The combination of pre- and post-TAVI MDCT scans showed that the deployed prostheses were overexpanded by >15% of the native annular area in 18 patients (19%). Interestingly, Edwards SAPIEN valves expanded to their nominal areas in only 2 patients, whereas in the rest of the patients, frames were underexpanded. On the post-TAVI scans, the mean depth of the deployed frame in the LVOT was 4.2 ± 2.4 mm.
The study end point (need for pacemaker implantation and new-onset LBBB) was observed in 15 patients (16%). As shown in Table 2 , the proportion of Edwards SAPIEN valves that were overexpanded by >15% of the native aortic annulus as assessed with MDCT was higher in patients who met the study end point compared with patients free of the combined end point (6 [40%] vs 12 [15%], p = 0.025). The frame was implanted deeper in the LVOT in patients who required new pacemakers or developed new LBBB compared with patients free of the combined end point (5.7 ± 2.7 vs 4.0 ± 2.3 mm, p = 0.014) ( Table 2 ). Binary logistic regression analysis showed that overexpansion of the valve by >15% of the native aortic annulus and depth of frame into the LVOT were independently associated with the study end point ( Table 3 ).
| Variable | Combined Endpoint (n = 15) | Free of Endpoint (n = 79) | p Value |
|---|---|---|---|
| Age (years) | 81 ± 4 | 80 ± 7 | 0.678 |
| Male | 9 (56%) | 37 (47%) | 0.492 |
| Creatinine (μmol/L) | 83 (57–97) | 88 (71–102) | 0.556 |
| Diabetes mellitus | 4 (25%) | 24 (30%) | 0.667 |
| Coronary artery disease | 11 (73%) | 54 (68%) | 0.702 |
| Coronary bypass | 5 (31%) | 19 (24%) | 0.648 |
| NYHA functional class III–IV | 9 (56%) | 47 (59%) | 0.810 |
| Beta-blockers | 8 (47%) | 45 (57%) | 0.609 |
| Transfemoral TAVI | 6 (38%) | 32 (41%) | 0.832 |
| Balloon post-dilatation | 2 (13%) | 7 (8%) | 0.865 |
| Edwards SAPIEN valve | |||
| 23 mm | 2 (13%) | 26 (33%) | 0.129 |
| 26 mm | 13 (86%) | 49 (62%) | 0.065 |
| 29 mm | 0 (0%) | 4 (5%) | 0.373 |
| Aortic valve area (cm/m 2 ) | 0.76 ± 0.16 | 0.71 ± 0.19 | 0.324 |
| Intra-ventricular septum thickness (cm) | 1.4 ± 0.3 | 1.4 ± 0.2 | 0.915 |
| MDCT data | |||
| AGATSTON score of aortic valve and LVOT (units) | 3026 ± 1765 | 2909 ± 1633 | 0.814 |
| LVOT ‘landing zone’ calcification (grade 1–4) | 2 (2–3) | 2 (2–3) | 0.440 |
| Overexpansion >15% of native annulus area | 6 (40%) | 12 (15%) | 0.025 |
| Depth of frame in LVOT (mm) | 5.7 ± 2.7 | 4.0 ± 2.3 | 0.014 |
| Preoperative heart rate (beats/min) | 73 ± 13 | 71 ± 11 | 0.446 |
| Preoperative atrial fibrillation | 3 (19%) | 11 (14%) | 0.629 |
| Preoperative PR (ms) | 188 ± 39 | 177 ± 21 | 0.203 |
| Preoperative QRS duration (ms) | 97 ± 10 | 98 ± 10 | 0.630 |
| Left axis deviation | 0 (0%) | 7 (9%) | 0.231 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


