Chapter 113
Infrainguinal Disease
Surgical Treatment
Joseph L. Mills Sr.,
Lower extremity arterial reconstruction is most commonly performed in patients with moderate to severe limb ischemia due to atherosclerotic peripheral arterial disease. Although the techniques described herein may also be applied to patients with traumatic, aneurysmal, and nonatherosclerotic conditions, this chapter focuses exclusively on patients with peripheral arterial disease. Infrainguinal bypass is defined as any major arterial reconstruction using a bypass conduit, either autogenous or prosthetic, that originates at or below the inguinal ligament. Inflow sites therefore include the common, deep, and superficial femoral arteries as well as the popliteal or even the tibial arteries. The bypass insertion site may be the femoral, above- or below-knee popliteal, tibial, peroneal, or pedal artery. During the past 2 decades, progress in patient evaluation and selection and in the conduct of infrainguinal bypass operations has resulted in a more aggressive and generally more successful approach to distal arterial reconstructions, especially for patients with critical limb ischemia (CLI) who would otherwise face major limb amputation. Although graft patency and limb salvage rates have demonstrated parallel improvements, there remains a critical need for detailed clinical studies examining the cost-effectiveness of infrainguinal bypass as well as patient quality of life outcomes to ensure the appropriate use of these procedures and to permit meaningful comparison with less invasive endovascular therapies.
Indications
The two primary indications for infrainguinal bypass are claudication and CLI.
Claudication
Patients who are significantly disabled by claudication, such that they are unable to perform their primary occupations or comfortably carry out the activities of daily living, and those whose lifestyles are significantly limited are potential candidates for infrainguinal bypass. A trial of smoking cessation, lifestyle modification, and exercise, with or without medical therapy, is usually indicated before operative intervention (see Chapter 109). There is consensus that bypass is preferable to angioplasty in patients with TransAtlantic Inter-Society Consensus (TASC) type D lesions, that is, complete common femoral artery (CFA) or superficial femoral artery (SFA) occlusions or complete popliteal and proximal trifurcation occlusions; however, it may also be applied to patients with types B and C lesions (see Chapter 109).1 Operation should be offered only if the benefit-to-risk ratio is high and if anatomic characteristics suggest a favorable and durable result. The primary reason for intervention in claudicants is to improve lifestyle, given that the risk of severe clinical deterioration (20%) or major limb amputation (5%) during a 3- to 5-year period is low.1,2 In most centers with extensive infrainguinal bypass experience, claudicants constitute only 15% to 30% of patients, with the majority of the remaining patients undergoing bypass for CLI. Although these data reflect practice patterns in tertiary referral centers, they may not reflect the realities of community-based practices.3
Critical Limb Ischemia
Patients with CLI generally require intervention. Such patients fall into Fontaine III and IV4 and Rutherford 4 to 6 categories (see Chapter 108).5–7 The most recent European consensus document defines CLI as follows: persistent, recurring ischemic rest pain requiring opiate analgesia for at least 2 weeks and ankle systolic pressure lower than 50 mm Hg or toe systolic pressure lower than 30 mm Hg; or ulceration or gangrene of the foot or toes and ankle systolic pressure lower than 50 mm Hg; or toe systolic pressure lower than 30 mm Hg (or absent pedal pulses in diabetics).8 Wolfe and Wyatt9 further subdivided patients meeting the standard definition of CLI into those with critical or subcritical ischemia on the basis of subsequent amputation risk. Subcritical ischemia is present in patients with rest pain and ankle pressure higher than 40 mm Hg. Critical ischemia is defined as rest pain and tissue loss or ankle pressure lower than 40 mm Hg. This distinction is based on a retrospective analysis of 20 publications analyzing 6118 patients. At 1 year, 27% of patients with subcritical ischemia achieved limb survival without revascularization, in contrast to only 5% in the group of patients with critical ischemia. In practice, these data indicate that certain extremely high risk patients with subcritical ischemia might be managed medically (nonoperatively) but that virtually all patients with true CLI require either bypass or major limb amputation.
Preoperative Assessment
There is widespread recognition that patients requiring infrainguinal bypass frequently have medical comorbidities, including diabetes mellitus, chronic obstructive pulmonary disease, and renal insufficiency; there is a particularly high prevalence of associated coronary artery disease (CAD). The incidence of perioperative myocardial infarction ranges from 2% to 6.5% after lower extremity arterial reconstruction; approximately 70% of both perioperative and late mortality in these patients is due to concomitant CAD.10–13 It is clear that significant CAD is a nearly universal accompaniment of peripheral arterial disease. What remains controversial is determining which individuals are most likely to benefit from a detailed preoperative cardiac assessment and possible coronary intervention before undergoing infrainguinal bypass. Proposed algorithms have ranged from routine cardiac evaluation of all patients with peripheral arterial disease to an almost nihilistic approach.14
Patients with CLI pose a more complex problem because there is a high anticipated amputation rate without lower extremity arterial reconstruction.9 CLI patients have an even greater prevalence of CAD than claudicants do as well as a significantly reduced 5-year mortality, primarily due to associated CAD. However, because CLI patients also tend to be older and have a greater number of comorbidities, cardiac intervention in such individuals has higher reported morbidity and mortality rates than in the general population of patients with isolated CAD targeted for intervention.14 My approach in CLI patients is to assume that they all have significant CAD. Perioperative blood pressure control, antiangina regimens, and treatment for congestive heart failure are optimized, and perioperative beta blockade is selectively employed if there are no contraindications.15–18 I would recommend postponement of infrainguinal bypass in CLI patients to allow further cardiac evaluation only in the presence of frequent or unstable angina, recent myocardial infarction, poorly controlled congestive heart failure, or symptomatic or untreated arrhythmia. Even in these instances, the cardiac evaluation should be focused and expeditious. Invasive coronary intervention should be pursued only if the patient and anatomic characteristics are favorable, the benefit-to-risk ratio is high, and the delay to perform the intervention is not prolonged. In the absence of such cardiac instability, CLI patients are best treated with meticulous perioperative medical care and expeditious lower extremity reconstruction.19,20 Prolonged delays before limb revascularization in CLI patients increase morbidity and amputation risk.21 In patients requiring vascular reconstruction, the focus has turned sharply toward maximizing medical therapy rather than performing multiple, expensive, and sometimes ambiguous preoperative cardiac tests so that the patient can be “cleared for surgery.” A large, well-designed prospective randomized trial failed to show a benefit of cardiac revascularization before vascular surgery in patients with stable cardiac symptoms.22 (See Chapter 31 for a detailed discussion of perioperative cardiac evaluation and treatment.)
Preoperative Imaging
Infrainguinal bypass requires a careful assessment of the extent of arterial disease as well as a detailed anatomic characterization of the inflow and outflow arteries (see Chapter 108). In most patients, standard arteriography is still the “gold standard.” Computed tomographic angiography has improved and is now the dominant preoperative imaging modality for aortic aneurysm disease; however, in the periphery, the small caliber of the infrainguinal artery and the presence of calcification in multiple vessels (associated with increasing age and diabetes) limit the applicability of computed tomographic angiography, especially in CLI patients.23 Magnetic resonance angiography24 is continually improving,25–29 and particularly for patients with claudication, preoperative duplex imaging, also referred to as duplex arterial mapping (see Chapter 16),30,31 may provide sufficient anatomic information to proceed directly to the operating room without formal arteriography. In this circumstance, immediate pre-bypass arteriography in the operating room is a reasonable approach. For most patients, however, especially for those with CLI, I prefer to perform the initial diagnostic angiography at a separate sitting, for several reasons. One reason is that for an increasing number of CLI patients, endoluminal techniques are a reasonable option.
On the basis of a careful assessment before angiography, including the indications for intervention, anatomic considerations (based on duplex imaging), and the patient’s functional status, comorbidities, and availability of vein conduit (see Chapter 109), I try to determine whether a given patient with TASC type C or D disease would be best served by endoluminal therapy or open bypass. If endoluminal therapy is the case, I vigorously pursue endoluminal treatment at the time of diagnostic arteriography. If open bypass is the plan, I focus on optimal visualization of potential target arteries and obtain multiple views, as required, to ascertain that no unexpected inflow disease is present that would require treatment before proceeding with infrainguinal bypass. Detailed high-quality diagnostic arteriograms can be obtained and reviewed carefully before proceeding with bypass. In some patients, selection of outflow arteries may be difficult, and this area of decision making can be improved if films are reviewed and discussed with colleagues. Confining the initial procedure to diagnostic or therapeutic angiography obviates time constraints and scheduling difficulties that arise in the operating room when trying to perform angiography and open reconstruction in one sitting. I reserve that approach for carefully selected patients whose arterial anatomy has already been fairly well delineated by preoperative magnetic resonance angiography or duplex scanning. This confines the “one-stop approach” primarily to claudicants undergoing bypass for isolated, single-level femoropopliteal disease.
Defining Bypass Target Arteries
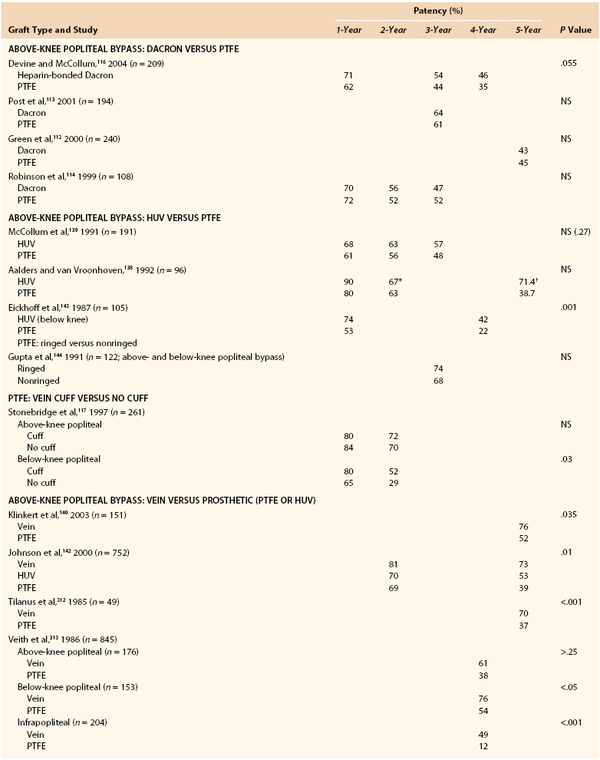
In nearly all patients in whom infrainguinal bypass is indicated, suitable target arteries can be identified (Fig. 113-1) if diagnostic angiography is properly performed.32,33 Only a tiny fraction of individuals, usually those with multiple failed reconstructions, have no identifiable target artery.34 Detailed runoff views are necessary, including magnification and lateral views of the foot. Such films can be obtained in nearly all patients with adjunctive techniques such as foot warming, local administration of intra-arterial vasodilators, and proper positioning of the diagnostic catheter. It is frequently helpful to advance the catheter selectively into the SFA or popliteal artery to obtain adequate views of the infrapopliteal and pedal circulations, especially in patients with diabetes mellitus. Intra-arterial runoff films with bolus injections performed while the diagnostic catheter is positioned in the aorta may fail to adequately define the runoff. If percutaneous access has been achieved from a contralateral, retrograde femoral approach, selective films can be obtained by advancing a wire and then an appropriate diagnostic catheter over the aortic bifurcation and selectively down the affected extremity. In selected patients with normal inflow based on physical examination and noninvasive studies, an ipsilateral antegrade approach may be more expeditious to identify suitable runoff vessels, with the additional advantage of requiring a reduced contrast load (Fig. 113-2). Such an approach is especially useful in diabetic patients with renal insufficiency and noninvasive studies that suggest isolated infrapopliteal disease.
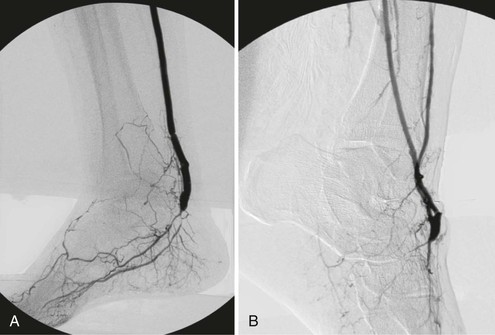
Figure 113-1 Detailed diagnostic arteriography with fixed imaging, proper timing, and appropriate catheter placement almost always identifies suitable target arteries. Each of the patients depicted had popliteal artery occlusion and extensive trifurcation and long-segment tibial disease, but diagnostic studies identified suitable target arteries in the foot. A, Completion arteriogram after inframalleolar posterior tibial bypass in a patient with diabetes and forefoot gangrene. Despite a small-caliber outflow vessel, the bypass remains patent, and the ischemic foot ulcers healed and have not recurred at 2 years. B, Completion arteriogram after bypass to a diseased dorsal pedal artery. Despite poor outflow and diseased arch and pedal vessels, the graft remains patent at 1 year. This patient with diabetes healed and ambulates with a transmetatarsal amputation.
Figure 113-2 Lateral foot view obtained by distal selective superficial femoral arterial catheter injection identifies excellent collaterals from the distal peroneal artery to both the dorsal pedal and posterior tibial circulations.
Despite optimal angiographic techniques, there may be a small number of patients in whom no suitable target can be identified. Selective exploration of dorsal pedal or distal posterior tibial arteries with flow detectable by Doppler or duplex imaging may identify a graftable recipient artery. Pomposelli et al34 reported a surprisingly high success rate under these circumstances, although I believe that this situation is relatively uncommon if diagnostic angiography has been pursued to its fullest advantage. In short, there are few patients who are truly unreconstructable from an anatomic standpoint owing to the lack of a suitable outflow target vessel.
Autogenous Vein Assessment
Since Kunlin’s first description of the successful use of autogenous vein to bypass femoropopliteal arteriosclerosis obliterans,35,36 there is nearly universal agreement that autogenous vein is the best conduit for infrainguinal bypass at all levels.37,38 The great saphenous vein (GSV) is the most readily available and durable conduit. Assessment of vein availability and quality is critical and should be carried out before embarking on the operation.39–41 I routinely perform duplex mapping of the GSV in all patients before surgery; if the ipsilateral GSV is absent, unsuitable, or of insufficient length for the anticipated bypass, I also scan the ipsilateral small saphenous vein, the contralateral great and small saphenous veins, and the upper extremity veins, if necessary, to locate a suitable vein and to identify any extenuating circumstances that might arise during the conduct of the operation. Patients are scanned with a light tourniquet in place with the limb dependent, as described by Blebea et al.42 I prefer to use veins that are soft, compressible, and at least 3 mm in diameter. Calcified or sclerotic veins are rejected. Soft, compressible veins between 2 and 3 mm in diameter are worthy of exploration, but if they do not distend appropriately, the operation should be modified either by harvesting a better-quality vein (based on preoperative duplex studies) or by shortening the length of the proposed bypass, if possible, by selecting alternative inflow or outflow sites. The type and quality of the bypass conduit are the most important determinants of infrainguinal bypass success; efforts to maximize conduit quality will be rewarded.
Vein harvesting can be performed through long continuous incisions, through skip incisions, or endoscopically. Endoscopic vein harvest has become common in cardiac surgery. Although there are a few enthusiasts, it has not been widely adopted by vascular surgeons for leg bypass, likely owing to equipment costs, learning curve issues, and concerns about damaging vein segments when a long conduit is needed. There are no convincing data to support any specific harvesting technique.43–47 Regardless of the harvesting technique, poor-quality or marginal vein should be rejected. The search for high-quality vein is worth the time and effort, even if vein splicing or bypass shortening is required. Every effort should be made to perform all infrainguinal bypasses with vein conduit (an all-autogenous policy). Long-term results should not be sacrificed for the sake of expediency at the initial operation. Saphenous vein performs well in the reversed,48–52 nonreversed,53 and in situ54–62 configurations; the technique chosen is dictated primarily by conduit availability, anatomic considerations, and surgeon preference and experience.
Operative Planning
Operative planning for infrainguinal bypass involves the most complex decision making a vascular surgeon is called on to provide. More than any other operation, infrainguinal bypass taxes the surgeon’s ingenuity and requires him or her to anticipate and carefully consider numerous alternatives and potential complications both in the preoperative evaluation and during the conduct of the reconstruction itself. Foremost, the major anatomic lesions and their hemodynamic significance must be identified.63–65
Concomitant Inflow Disease
Adequate inflow should be ensured before commencing with infrainguinal bypass. Selected inflow lesions can be treated either percutaneously in advance, at the time of preoperative diagnostic arteriography, or at the same operative sitting if need be. Iliac artery lesions of hemodynamic significance should be addressed in nearly all claudicants before proceeding with infrainguinal bypass. For patients with CLI, an iliac lesion with a resting gradient of less than 5 to 10 mm Hg may be acceptable if the pulse and Doppler waveform at the selected inflow site (e.g., femoral artery) are normal. In patients with claudication or CLI presenting with rest pain alone in the absence of tissue loss, an isolated iliac angioplasty without concomitant infrainguinal bypass may suffice if the iliac lesion is of sufficient hemodynamic importance. In such patients with tandem lesions, the profunda-popliteal collateral index may be helpful in predicting whether an inflow procedure alone will be sufficient to alleviate the patient’s symptoms.63 An index greater than 0.25 indicates a large pressure gradient across the knee joint and suggests that inflow disease correction and profundaplasty alone are unlikely to be adequate.66–68
Proximal Anastomotic Site
Before operation, the surgeon must define the inflow source, which need not be the CFA. There is abundant evidence that originating shorter bypasses from the deep femoral, superficial femoral, popliteal, or, in rare cases, one of the tibial arteries results in patency rates equivalent to those achieved when the CFA serves as the bypass origin. Short bypasses are frequently useful in patients with diabetes mellitus and primary infrapopliteal arterial occlusive disease as well as in individuals with limited available vein conduit who present with failure of a previous reconstruction. On the basis of hemodynamic data and anatomic imaging, the surgeon should commence the operation with the optimal inflow site in mind. However, if unanticipated arterial disease is identified or vein quality and length are worse than anticipated once the operation is under way, the surgeon should already have alternative bypass origins in mind that can be used to shorten the length of the bypass without compromising hemodynamics. If there is uncertainty about the appropriateness of the inflow site at exploration, its hemodynamic suitability should be assessed by direct intra-arterial pressure measurements that can be compared with the transduced radial artery pressure. A resting gradient exceeding 10 mm Hg is significant, as is a drop in pressure exceeding 15% after the administration of intra-arterial papaverine.64,65,69 If a significant gradient is identified at the selected inflow site, a more proximal inflow site above the culprit lesion should be selected, or the responsible lesion should be addressed by local endarterectomy or angioplasty. This problem occasionally arises with iliac or common femoral lesions whose hemodynamic significance was not appreciated at the time of preoperative arteriography, particularly lesions consisting primarily of posterior plaque that may have been masked if appropriate oblique projections were not obtained.
Associated Femoral Endarterectomy
Whenever the CFA is used as the site of origin for bypass, significant occlusive disease involving the origin and proximal deep femoral artery should usually be addressed at the time of the infrainguinal bypass. The endarterectomy often begins in the CFA. After the division of veins that cross the anterior surface of the deep femoral artery, the femoral arteriotomy is carried out beyond the posterior tongue of disease that extends a variable distance down the deep femoral artery, usually at least to its first or second portion. Tacking sutures may or may not be needed at the distal endpoint. The arteriotomy can be closed with a vein patch or a segment of endarterectomized SFA. This patch can then be opened longitudinally to serve as the origin for the infrainguinal bypass (modified Linton’s patch technique; Fig. 113-3). Alternatively, if the vein caliber is good (>4 mm), a longer venotomy can be made in the vein conduit, which then serves simultaneously as a profundaplasty patch and the bypass origin; this technique should not be used if the arterial wall is markedly thickened or if the caliber of either the native donor artery or the vein graft artery is small to avoid compromising the origin of the vein graft at the heel of the anastomosis. Incorporation of a venous side branch as part of the anastomotic heel is another useful technique when the donor arterial wall is thick or there is a caliber mismatch between the donor artery and the vein bypass conduit (Fig. 113-4). Correction of significant deep femoral disease at the time of infrainguinal bypass is clinically important; should the bypass ever fail, adequate deep femoral artery perfusion may prevent the development of severe, recurrent limb ischemia.
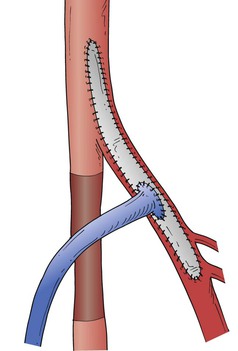
Figure 113-3 Linton’s vein patch technique after endarterectomy of the distal common and proximal deep femoral arteries, with anastomosis of the reversed vein graft to the patch.
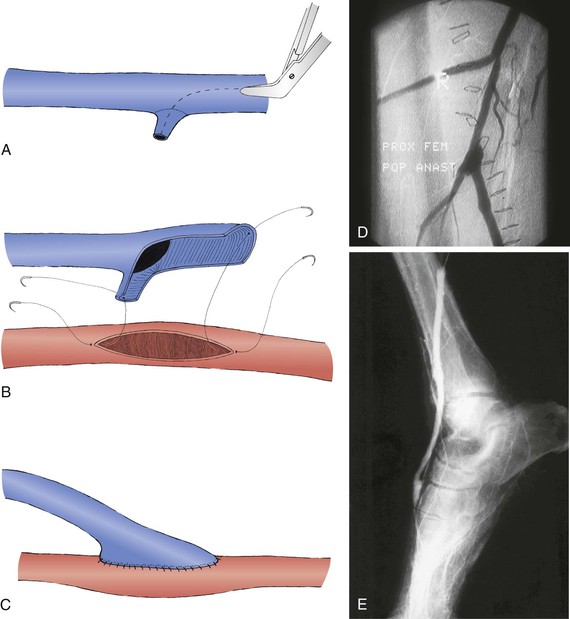
Figure 113-4 The venotomy through the reversed vein is extended through a suitable side branch (A) and anastomosed to the inflow artery (B and C) to avoid anastomotic stenosis at the graft heel.35,314 This technique can be used at either the proximal anastomosis (D) or the distal anastomosis (E) and is useful when the vein caliber is small or the arterial wall is thickened.
Distal Anastomotic Site
Although inflow artery selection is generally straightforward, outflow site selection frequently requires greater judgment. The general principle of infrainguinal reconstruction is to bypass all hemodynamically significant disease and to insert the bypass into the most proximal limb artery that has at least one continuous runoff artery to the foot. Thus, if the popliteal artery reconstitutes distal to an SFA occlusion and at least one tibial or peroneal artery is continuous to the foot, the popliteal artery would be selected as the outflow site. It is possible, however, and sometimes desirable to bypass to an isolated or so-called blind popliteal segment.
Isolated Popliteal Target
An isolated popliteal artery is defined as a patent popliteal artery segment at least 5 cm long but with only geniculate collaterals and no major distal tibial or peroneal runoff artery in direct continuity with the foot. Such bypasses function surprisingly well and are especially useful in patients with limited vein availability.70–75 They may also be more useful in patients with claudication or rest pain than in those with frank tissue necrosis, for whom anything less than providing pulsatile flow directly to the foot may be insufficient. In the presence of tissue loss, I prefer bypass to an artery in direct continuity with the foot. Five-year assisted primary or secondary patency rates of 50% to 74% have been reported for such blind-segment popliteal bypasses.73 Successful bypasses to isolated tibial artery segments have also been reported.71
Tibial, Peroneal, and Pedal Targets
Although most claudicants require only femoropopliteal bypass, a high proportion of patients with CLI require tibial or pedal bypass. In general, the most proximal segment of tibial or peroneal artery that is continuous with the foot should be chosen as the outflow site. Thus, a patent anterior tibial or posterior tibial artery in direct continuity with the foot and pedal arch would be chosen over the peroneal artery as an outflow site if suitable vein length is available. There is still some controversy about whether one should choose the proximal or mid peroneal artery or a patent pedal artery for patients with tissue loss.76 Most authors have found no adverse effects on graft patency or limb salvage for peroneal bypasses compared with tibial or pedal bypasses,77–79 but Pomposelli and others have made a strong case for pedal bypass,34 particularly in diabetic patients with tissue loss.80 They emphasize the importance of restoring a pedal pulse and maximizing forefoot reperfusion. The pedal arteries are also more superficial and more easily exposed than the anatomically disadvantaged, deeply located peroneal artery. If the bypass must originate in the groin and the proximal or mid peroneal artery is of good quality on arteriography and has abundant collaterals with the foot (see Fig. 113-2), I generally perform a shorter bypass to the peroneal artery, especially if vein conduit length is a limiting factor. If the peroneal artery is diseased or does not appear to collateralize well with the foot, I preferentially bypass to the foot or ankle and splice a vein if it is required to obtain sufficient length. I prefer dorsal pedal or paramalleolar posterior tibial–plantar artery insertion sites for short bypasses originating from the popliteal artery in diabetic patients with tissue loss.81 Shortening the bypass in such patients allows one to optimize vein conduit quality, and the choice of an inframalleolar target artery maximizes forefoot perfusion.
Operative Exposures
Standard Anterior Approach to the Common Femoral and Profunda Femoris Arteries
A thorough knowledge of anatomy and facility with multiple surgical exposures are critical to the success of infrainguinal bypass.82 The standard approach to the CFA and the profunda femoris artery (PFA) is provided by a vertical incision overlying the CFA. This anterior approach allows complete exposure and mobilization of the CFA and its bifurcation; more proximal exposure can be obtained by division of the recurrent portion of the inguinal ligament or the entire ligament (Peter Martin incision). Distal extension allows exposure of the PFA. Division of the lateral femoral circumflex vein offers access to the proximal PFA; careful progressive division of numerous crossing veins allows extensive exposure of the PFA. In selected patients, alternative exposures have been described.
Alternative Approaches to the Profunda Femoris Artery
If previous infection or scarring from multiple previous reconstructions makes standard exposure difficult and if there are no significant occlusive lesions in the CFA or proximal PFA, the mid or distal PFA can be approached laterally, anteromedially, or posteromedially (Fig. 113-5).71,83–86 The lateral approach is the most useful for infrainguinal bypass; the incision is placed in the upper thigh lateral to the sartorius muscle. The sartorius and SFA are retracted medially. The raphe between the adductor longus and vastus medialis is incised to expose the PFA. This approach is useful when vein conduit length is limited or femoral triangle scarring is prohibitive. The surgeon must be certain that there are no hemodynamically significant lesions proximal to the PFA if this approach is used. With these caveats in mind, use of the PFA as an origin for distal bypass does not compromise long-term patency85,86; similarly, the SFA87 and popliteal artery85–88 can be used as an inflow source in carefully selected patients without compromising graft patency.
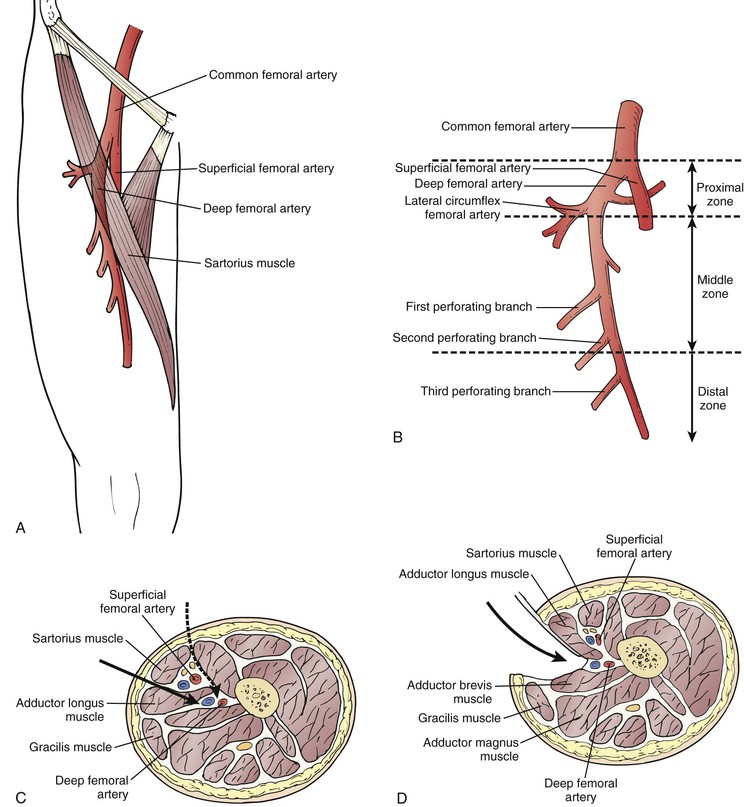
Figure 113-5 Alternative approaches to the distal deep femoral artery85 are useful for reoperative femorodistal bypass and when it is desirable to shorten the bypass because of limited length of vein conduit.86 A, Location of the deep femoral artery. Note the surface landmarks used to identify its course. B, The deep femoral artery can be divided into three zones: proximal, middle, and distal. C, Transverse section of the thigh (viewed from above) shows the plane of dissection when the anteromedial approach (solid arrow) is used. Alternatively, the deep femoral artery can be approached through an even more anterior route (dashed arrow) by making an incision along the lateral border of the sartorius and retracting this muscle and the superficial femoral neurovascular bundle medially to reach the distal deep femoral artery. D, Posterior approach to the distal deep femoral artery. Note the fascial plane and structures separating the deep femoral artery from superficial femoral vascular structures and isolating it from the subsartorial canal.
Exposures of the Popliteal and Tibioperoneal Arteries
The standard exposures of the above-popliteal and below-knee arteries are through medial leg incisions, usually made by deepening the saphenectomy incision. Lateral approaches to these vessels are occasionally useful and are well described elsewhere.88 The posterior tibial and proximal to mid peroneal arteries are usually approached medially. The distal third of the peroneal artery is most expeditiously exposed by means of a lateral leg incision directly over the distal fibula. A short segment of fibula is carefully removed to expose the distal peroneal artery immediately beneath it.
Posterior exposures of the popliteal, posterior tibial, and peroneal arteries are sometimes useful (Fig. 113-6).89,90 Diabetic patients frequently have relatively normal inflow to the popliteal trifurcation. If the inflow site is the below-knee popliteal artery and the most appropriate target artery is the distal posterior tibial or distal peroneal artery, and if the lesser saphenous vein is of good quality on duplex imaging, the entire operation can be conducted with the patient in a prone position through a posterior approach. These approaches have been well described by Ouriel90 and should be considered not only in selected reoperative situations (e.g., failed bypass through a medial approach) but also if conduit length is limited and ipsilateral GSV is unavailable. Commitment to an all-autogenous bypass approach requires ingenuity and familiarity with numerous anatomic exposures and techniques to allow shortening of the bypass or to permit operation in a virgin field that is unscarred by previous operations. When such distal origin bypass grafts are used, a pneumatic tourniquet instead of vascular Silastic loops or clamps is occasionally useful in the presence of severe distal arterial calcification.91 A thorough working knowledge of alternative exposures and the occasional use of the pneumatic tourniquet are of great benefit in performing reoperative bypass.
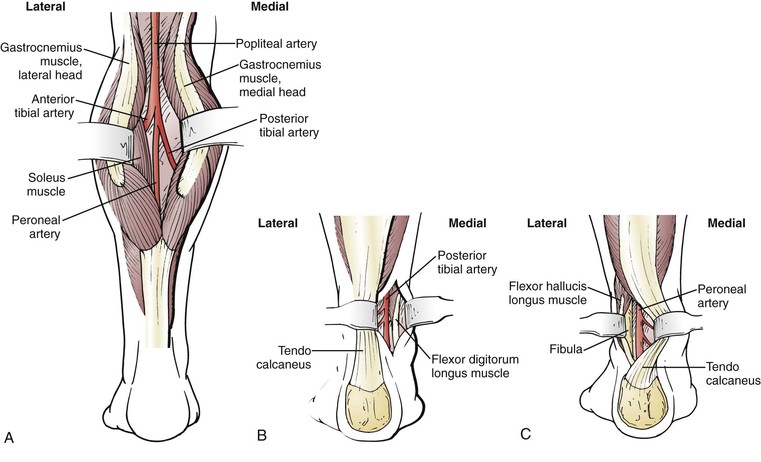
Figure 113-6 Posterior exposures.315 A, Exposure of the popliteal and proximal crural arteries through a posterior approach. The two heads of the gastrocnemius muscle are separated, and the soleus muscle is lysed from its tibial origin. B, Exposure of the posterior tibial artery through a posterior approach. The tendo calcaneus is retracted laterally. The flexor digitorum longus muscle is reflected medially in the lower portion of the calf because the artery lies posterior to the lateral border of this muscle near the level of the malleoli. C, Exposure of the peroneal artery through a posterior approach. The tendo calcaneus is retracted medially and the flexor hallucis longus muscle is reflected laterally to expose the artery in the groove next to the fibula.
Choice of Conduit
Autogenous conduit options for infrainguinal bypass include ipsilateral and contralateral GSV,92 small saphenous vein,93,94 superficial femoral vein,95 arm (basilic and cephalic) vein,96–101 endarterectomized segments of the SFA,102 cryopreserved vein,103–105 and radial artery.106,107 Prosthetic options include Dacron, heparin-bonded Dacron, human umbilical vein, polytetrafluoroethylene (PTFE) with and without a distal cuff, and, most recently, expanded PTFE (ePTFE) covalently bonded with heparin. In my group’s reported 10-year experience, we performed 93% of infrainguinal bypasses with all-autogenous conduits.108
Autogenous Grafts
The preferred conduit is the GSV as it outperforms all other conduit choices. If the ipsilateral GSV is absent, I do not hesitate to harvest the contralateral GSV and see no merit in saving this vein for possible use later.92 Numerous reports suggest that the contralateral GSV is subsequently needed in no more than 20% to 25% of patients. I therefore advocate using it when necessary and performing a more difficult alternative vein reconstruction at a later time in those relatively few patients who require it. Exceptions occur whenever the contralateral limb is already ischemic, as manifested by severe claudication, rest pain, or ischemic ulceration. If the contralateral limb is asymptomatic and the ankle-brachial index exceeds 0.6, I have experienced no significant wound healing complications from harvesting the GSV from the groin to the midcalf level. This approach usually allows the bypass to be performed with one segment of GSV and obviates the harvesting of arm vein and vein splicing. Other groups prefer to harvest arm vein if the ipsilateral GSV is unavailable and to save the contralateral GSV for later use.96,109
Alternative veins are used when the GSV is unavailable or of insufficient length.110 Duplex mapping is useful in identifying suitable vein sources. The small saphenous vein is suitable if the proposed bypass is relatively short. It is possible to perform a common femoral–to–above-knee popliteal bypass or a PFA–to–below-knee popliteal bypass with one complete segment of lower saphenous vein harvested from the ankle to the knee. If a longer bypass with spliced vein is necessary, I prefer to use arm vein because it is less awkward to harvest. LoGerfo et al111 and Holzenbein et al100 described novel techniques of harvesting the upper arm basilic, median cubital, and cephalic veins in continuity with valve lysis of the basilic segment and use of the cephalic segment in a reversed configuration to provide a relatively long, unspliced autogenous conduit. The femoropopliteal deep vein is occasionally useful for shorter bypasses but is difficult to harvest; arm vein is therefore generally preferred. Treiman et al107 reported the use of the radial artery as a bypass conduit in selected patients requiring short infrageniculate bypasses, with good early results. Cryopreserved vein grafts are expensive and have not performed well in clinical practice103,104; they may serve a niche role when revascularization is required after the removal of an infected bypass graft and autogenous vein is unavailable to create a new bypass through clean tissue planes.104
Prosthetic Grafts
PTFE is the most commonly used prosthetic conduit for infrainguinal bypass, although reports suggest that in the above-knee position, it is not superior to Dacron.112–114 A prospective randomized trial from the United Kingdom reported by Devine et al115 suggested that heparin-bonded Dacron was superior to PTFE for above-knee popliteal bypasses. The 3-year primary patency for heparin-bonded Dacron was 55% compared with 42% for PTFE (P < .044). Both of these patency rates are inferior to those of GSV, however, and the early apparent advantage of heparin-bonded Dacron over PTFE disappeared with longer follow-up.116
Vein Cuffs
For bypasses that insert below the knee, the addition of a vein cuff confers a significant patency advantage (52% patency at 2 years for PTFE with vein cuff vs 29% for PTFE with no cuff) and also improves limb salvage (84% vs 62%; P < .03).117 My experience and that of others suggests that a distal vein cuff or collar results in improved 2- to 3-year patency for infrageniculate bypass when PTFE is required.118–122 Nevertheless, the results are still inferior to those of vein bypasses, even when alternative veins are used; these data emphasize the validity of the all-autogenous policy. If vein is truly limited, however, PTFE is an acceptable choice, and available data suggest that distal anastomotic modification with autogenous tissue is a worthwhile adjunct. The only prospective randomized clinical trial used a Miller cuff (Fig. 113-7).117 A distal Taylor patch (Fig. 113-8)118,120 and the St. Mary’s boot (Fig. 113-9)122 may yield equivalent results. Neville et al123,124 reported an alternative PTFE adjunct consisting of anastomosis of a PTFE graft to an infrageniculate artery using a vein patch, analogous to the technique originally described by Linton. At least in their hands, this adjunct seems to work as well as a Miller cuff or Taylor patch. However, the only randomized prospective trial of such adjuncts used the Miller cuff, and this trial has not been repeated to my knowledge.
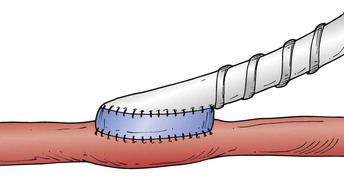
Figure 113-7 The Miller cuff technique119,316 improved the patency of below-knee popliteal and tibial polytetrafluoroethylene bypass grafts when used as a distal anastomotic adjunct in a controlled randomized clinical trial.117
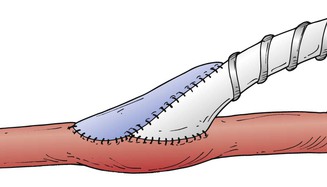
Figure 113-8 The Taylor patch technique can improve the patency of infrageniculate polytetrafluoroethylene bypass grafts.118,120
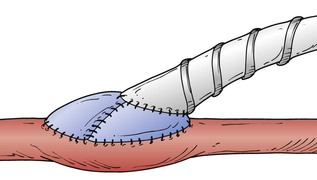
Figure 113-9 The St. Mary’s boot or prosthetic venous collar technique122 combines the attributes of the Taylor patch and the Miller cuff.
Human Umbilical Vein and Adjunctive Arteriovenous Fistula
Human umbilical vein is less commonly employed than PTFE, primarily because it is thicker and more cumbersome to handle and because of concerns about subsequent aneurysmal degeneration.125 Dardik et al126 reported excellent results using human umbilical vein with an adjunctive distal arteriovenous fistula to promote increased graft flow velocity; there are no prospective trials comparing this technique with alternative prosthetic conduits, such as PTFE. A single prospective trial did not demonstrate any benefit of the addition of an arteriovenous fistula to femoral-infrapopliteal bypass with vein cuff.127
Heparin-Bonded PTFE
The most recent addition to the inventory of prosthetic infrainguinal bypass conduits is ePTFE to which heparin has been covalently bonded. The commercially available product was originally called Carmeda BioActive Surface (CBAS).128 Preclinical studies suggest that biologically active heparin has been successfully covalently bonded to ePTFE without causing systemic anticoagulative effects.129 The drug is biologically active, at least in baboons and dogs, for up to 12 weeks.130 In animal models of thromboresistance (short 3- and 4-mm grafts), CBAS appears to prevent early ePTFE graft thrombosis; this effect may persist up to 180 days, although not as strikingly. It also appears to reduce early platelet and fibrin deposition in animal models.
There is no proof, however, that these experimental findings will translate into improved prosthetic graft patency in humans. The five published reports of CBAS for peripheral infrainguinal bypass suffer from major flaws, including lack of randomization, lack of control groups, small numbers of patients, selection bias, large numbers of patients with relatively prosthetic-friendly above-knee popliteal insertion sites, lack of intermediate follow-up (beyond 2 years), and flawed or unreliable life tables.128,131–134 In two of these five series, anastomotic adjuncts such as patches were used in addition to CBAS grafts, further confounding interpretation. In addition, aggressive combination antiplatelet therapy (oral and low-molecular-weight heparin) was used by nearly all the authors; this combination is not routinely used in North America.
If one assumes that there is no patient overlap between the two studies from the same center (an assumption that may well be false),131,134 the entire published human experience with CBAS infrainguinal grafts consists of 356 grafts with the following insertion site distribution: 142 (41%) above-knee popliteal, 115 (33.5%) below-knee popliteal, 75 (21.8%) femorocrural or tibial, and 24 indeterminate (one study does not specify target arteries).135 The estimated number of patients available with documented follow-up in all five human clinical CBAS studies combined is as follows: 95 above-knee popliteal, 45 below-knee popliteal, and 25 femorocrural at 1 year; and 48 above-knee popliteal, 16 below-knee popliteal, and 9 femorocrural at 2 years. The data and follow-up length are thus insufficient to support assertions of improved patency conferred by covalently bonded heparin grafts. Long-term studies of this intriguing bioactive graft are needed to determine whether covalent heparin bonding confers any significant patency benefit. Heparin-induced thrombocytopenia has also been reported with the use of these grafts.
Graft Comparison
Table 113-1 summarizes the available level I data comparing conduit types with outcome.114–120,127,136–140 Vein is superior to all prosthetic materials, even in the above-knee position.140–142 The randomized clinical trials comparing human umbilical vein with PTFE have been inconclusive.138,139,143 The addition of rings to PTFE conferred no benefit in the single prospective randomized clinical trial conducted.144 Thus, the most prudent recommendation is that autogenous vein be used whenever possible for infrainguinal bypass. This dictum holds true not only for primary infrainguinal bypasses but also for reoperations, in which vein conduits outperform all other options.145,146 For long bypasses, ipsilateral GSV, contralateral GSV, and spliced vein are employed in decreasing order of preference. If only 5 to 15 cm of extra length is required and a more distal origin site is not feasible, eversion endarterectomy of a proximal segment of the SFA with anastomosis to the available vein conduit is a useful technique that avoids the harvesting and splicing of additional vein (Fig. 113-10).102 For shorter bypasses, arm vein or small saphenous vein is effective, with the latter being especially useful when a posterior approach is applicable.
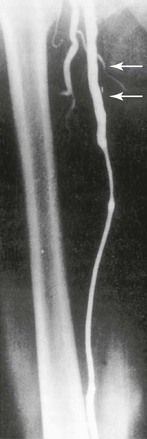
Figure 113-10 When vein length is limited, an eversion endarterectomy of a proximal superficial femoral artery segment (arrows) can be performed.102 The everted arterial segment is anastomosed to the available vein graft to create an all-autogenous conduit of sufficient length for the required bypass to be performed.
If vein is truly unavailable, PTFE, human umbilical vein, or Dacron is a reasonable option for above-knee bypass. The initially favorable results with heparin-bonded Dacron were not confirmed during subsequent follow-up,115,116 so there appears to be no advantage to heparin-bonded Dacron conduit for above-knee bypass. A prospective randomized trial of human umbilical vein versus heparin-bonded Dacron showed no difference in patency in the above-knee popliteal position,147 and published trials of PTFE versus Dacron have shown no clear difference.112–114,116 For infrageniculate insertion sites, PTFE with distal anastomotic modification (cuff, boot, or patch) is recommended if vein is unavailable. There is inadequate evidence to state a preference in terms of standard ePTFE versus heparin-bonded or carbon-coated PTFE. The single prospective randomized trial of standard PTFE versus carbon-impregnated PTFE for infrageniculate bypass showed approximately 30% primary patency, with no difference between groups at 3 years.148
Bypass Technique
Reversed, nonreversed, and in situ conduits appear to work equally well. The choice is primarily one of surgeon preference. Arm veins pose special challenges. Regardless of which type of conduit is used, meticulous technique and gentle handling of the vein are critical.
Reversed Vein Bypass
Harvest of the GSV begins with a groin incision two fingerbreadths lateral to the pubic tubercle. Preoperative duplex vein mapping and drawing of a line with indelible ink directly over the vein conduit are helpful in following the course of the vein and avoiding the creation of skin flaps. The saphenofemoral junction at the fossa ovalis should be identified to ensure that the GSV and not an anterior or large posteromedial branch is being exposed. Once the main trunk is identified, the incision is extended distally, directly over the vein, with a No. 10 blade or Cooley scissors. I frequently leave short skin bridges, especially in the thigh and about the knee level, to avoid the creation of long flaps. If exposure or branch ligation is difficult or if the course of the main vein is unclear, the skin directly over the vein is divided. The periadventitial tissue, not the vein wall itself, is grasped gently to avoid damage to the conduit. Side branches are meticulously ligated and divided with 3-0 or 4-0 silk; leaving a short stump is preferable to placing a ligature too close to the main trunk and compromising the lumen. When it is first exposed, the vein should be soft and blue. Sclerotic segments are whitish and rubbery. Vein spasm can turn a blue vein white and should be treated with local papaverine irrigation.
Sufficient length of vein should be harvested for the proposed bypass to be performed. If adequate vein of good quality is not available, alternative inflow or outflow arteries should be considered to shorten the bypass; otherwise, alternative vein must be harvested from another site and spliced on a back table. Vein quality is extremely important, and segments less than 3 mm should not be used. Once an adequate length of vein is identified, it is ligated proximally and distally and prepared on the back table by a senior surgeon while the rest of the team exposes the inflow and outflow arteries.
The vein is cannulated with a 3-mm olive tip or Marx tip needle and gently distended with cooled autologous blood (50 to 60 mL) to which 1000 units of heparin and 60 mg of papaverine have been added. The vein should flow readily, should distend smoothly, and should be free of fibrotic, nondistensible segments, strictures at branch ligature sites, and bleeding from missed branches. Missed or small avulsed branches are repaired with longitudinally oriented 6-0 or 7-0 Prolene sutures on a BV-1 or BV-175 needle. Overly forceful distention of the conduit should be avoided because it damages the endothelium. The entire length of the conduit is inspected to ensure that there are no sclerotic areas, no areas of persistent spasm, and no bleeding branches. Once preparation is complete, the distal end of the vein is ligated with 3-0 silk, leaving the suture ends long; the prepared conduit is stored in chilled blood until the graft is ready to be tunneled. Proper intraoperative planning should result in minimal vein storage time. Many believe that autologous blood, rather than saline, better preserves graft endothelium.149
Proper graft tunneling of reversed grafts is important. For primary leg bypasses with GSV, I prefer to tunnel the graft in a deep anatomic plane to avoid kinking and graft exposure should wound complications ensue. For above-knee popliteal bypasses, a subsartorial plane is frequently easier than a true anatomic tunnel. A large-caliber hollow-bore metal tunneler with a removable obturator is used. For below-knee popliteal and proximal to mid posterior tibial or peroneal bypasses, I generally prefer an anatomic tunnel between the two heads of the gastrocnemius for initial bypasses. It is often wise to tunnel reoperative bypasses subcutaneously; this approach avoids scarring and makes graft surveillance and revision easier, which is advantageous because the alternative vein conduits often used in these circumstances are at higher risk for the development of graft stenosis. Bypasses to the anterior tibial artery can be tunneled through the interosseous membrane (anatomic) or a lateral subcutaneous plane.
Arm Vein Harvest and Vein Splicing
If leg vein length is inadequate or unavailable or if additional conduit is required, arm vein should be used. Arm veins, especially the basilic vein, are more fragile and demanding to harvest. Branches should be carefully identified and ligated a short distance away from their junctions with the main trunk to avoid troublesome bleeding from an injury at the crotch between the branch and the parent vein. Large branches are double-ligated. The initial irrigation and distention of the vein are performed with heparinized saline and papaverine because defects in thinner walled arm veins are more difficult to visualize and to repair if blood is used as the initial irrigant. Once major leaks have been controlled, the vein graft is prepared and stored in chilled blood as mentioned earlier. It is worth considering that arm veins frequently harbor abnormal segments containing intrinsic lesions such as webs, synechiae, and sclerotic valves. Angioscopy of such high-risk conduits may be a useful adjunct (see later under Angioscopy).
In Situ Vein Bypass
In situ bypass can be used successfully in many patients with intact ipsilateral GSVs. Proponents suggest that the vein-artery size match is frequently optimized by this technique.150 This technique requires the use of a radial cutting blade (Mills valvulotome)56 or fixed-diameter circumferential blades such as the Hall57 and LeMaitre valvulotomes to accomplish valve lysis.60,151 The proximal segment of GSV is initially mobilized in the groin as it would be for reversed vein bypass, described earlier. The distal GSV is then mobilized in the region of the projected distal arterial anastomosis. The vein may be exposed by an open technique along its entire length, through skip incisions, or endoscopically. After systemic heparinization, the vein is transected below a Satinsky clamp placed at the saphenofemoral junction, and the stump is closed with running 5-0 Prolene suture. The most proximal valve is excised under direct vision with Potts scissors, and the vein is spatulated and sewn end to side to the selected arterial anastomotic site, usually the CFA. Arterial flow is allowed into the vein conduit; if the conduit is of large caliber, a circumferential valvulotome can be inserted from distal to proximal to lyse the valves. Many surgeons prefer to more carefully control valve lysis by progressive serial valvulotomy through large side branches, progressing from proximal to distal. The Mills valvulotome is introduced through side branches; the leaflets are usually just distal to branches and are oriented parallel to the skin. Under arterial pressure, the valve sinuses distend, and the valve leaflets are displaced toward the center of the lumen, where they can be safely engaged by the valvulotome and cut by sharp retraction on the valvulotome under direct visualization. The final valves are lysed from the distal end of the vein. The quality of the vein and the success of lysis are then assessed; flow should be highly pulsatile from the end of the conduit before the distal anastomosis is performed.
After completion of the anastomoses, the quality of pulsation and flow is assessed by palpation and handheld Doppler interrogation. The entire graft should be evaluated from proximal to distal; it can be manually compressed or occluded distal to the Doppler probe. The presence of continuous outflow despite distal graft compression indicates an arteriovenous fistula; these branches should be ligated. With either the reversed or the in situ technique, careful handling of the vein is important. The assurance of adequate valve lysis and the detection of major arteriovenous fistulae can be challenging; the type of completion study used depends on the type, source, and quality of the conduit and surgeon preference.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree