Chapter 114
Infrainguinal Disease
Endovascular Treatment
Reid A. Ravin, Peter L. Faries
Based on a chapter in the seventh edition by Mikel Sadek and Peter L. Faries
Endovascular treatment is often the first option for treating infrainguinal peripheral arterial disease (PAD). Percutaneous transluminal angioplasty (PTA) with adjunctive stenting is an increasingly used and accepted technology, and it is the technique most frequently employed for infrainguinal endovascular treatment. An alternative to transluminal angioplasty is subintimal angioplasty (SIA), also referred to as percutaneous intentional extraluminal revascularization. SIA is used to cross occlusive lesions, and recanalization is performed by intentionally exiting the vascular lumen, entering the subintimal plane, and then reentering the vascular lumen distal to the lesion. The diseased arterial segment is subsequently balloon-dilatated in the subintimal plane to achieve revascularization.1 Stenting is an adjunctive procedure used either routinely or selectively for the treatment of complex lesions or persistent stenosis or to correct intraprocedural complications. Recently, drug-eluting stents (DES) have been designed that release antiproliferative drugs after implantation.
Early experiences with lower extremity arterial PTA were characterized by poor immediate and long-term outcomes.2,3 Factors contributing to these poor results included the presence of long-segment disease, chronic occlusion, and compromised distal outflow. Endovascular therapy of infrainguinal PAD has since gained acceptance owing to reported improvements in outcome and diminished rates of morbidity and mortality compared with standard surgical bypass.4–6 Novel technologies and refinements of previous technologies are enabling endovascular therapy for increasingly complex vascular pathology. Patients with multiple comorbidities or those lacking adequate autogenous conduits may derive particular benefit from an endovascular approach, because the utility of standard bypass is more limited in these cases.7
This chapter focuses on the measures of success for infrainguinal endovascular treatment, the factors that influence procedural outcome, and the results from recent clinical trials that demonstrate the safety and efficacy of infrainguinal endovascular therapy. For additional information on preoperative testing, planning, and specific endovascular techniques, refer to Chapters 89, 108, and 109.
Endovascular Planning
Most studies demonstrate that infrainguinal endovascular therapy can be carried out with limited periprocedural risk.8,9 Nevertheless, the decision to proceed with infrainguinal endovascular therapy rather than an open surgical approach requires a thorough risk-benefit analysis based on the information acquired from the history, physical examination, noninvasive imaging, and diagnostic angiography. Salient features of the history and physical examination, when planning an intervention, include (1) indication (claudication versus critical limb ischemia [CLI]); (2) disease location, extent, and severity; (3) degree of disability and lifestyle limitations; (4) medical comorbidities and anesthetic risk; (5) prior lower extremity reconstructions or interventions; and (6) prospects for long-term functional status and survival. For detailed discussions of duplex ultrasonography, computed tomographic angiography (CTA), magnetic resonance angiography (MRA), and digital subtraction angiography, refer to Chapter 108. Additional variables that may influence the decision-making process include the expertise of the treating physician, the consequences of endovascular treatment failure, and the long-term costs of care.
Treatment Guidelines
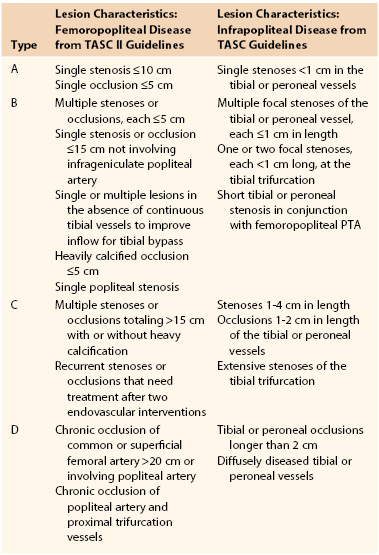
Guidelines for infrainguinal endovascular therapy have evolved with the accumulation of experience and data. In 1994, the American Heart Association (AHA) Task Force compiled a list of recommendations for the use of PTA in the infrarenal vasculature.10 The anatomic criteria were divided into four categories of increasing severity, based on the premise that milder disease should be managed with PTA and more complex disease should be managed with surgical revascularization. The AHA Task Force criteria were formulated in general terms and relied on a body of evidence collected before the routine use of stenting. Improvements in reporting standards, longer postintervention follow-up, advances in technique, and the more prevalent use of adjunctive technologies such as stenting have contributed to improved outcomes and consequently to refinements in the guidelines for endovascular therapy of infrainguinal PAD.11,12 The Trans-Atlantic Inter-Society Consensus (TASC) guidelines released in 2000 and the revised TASC (II) guidelines released in 2007 are the most broadly accepted anatomic classification system.12 These guidelines distinguish the treatment of aortoiliac, femoropopliteal, and infrapopliteal disease. The revised TASC criteria did not include a classification system for infrapopliteal lesions, but the original infrapopliteal TASC classification system is still in wide use. Future TASC updates will likely present a refined infrapopliteal classification system and recommendations, reflecting the evolution of complex infrapopliteal endovascular interventions.
Femoropopliteal
The TASC II femoropopliteal criteria reflect the fact that increasingly complex disease can be treated using endovascular techniques (Table 114-1). TASC type A lesions are suitable candidates for endovascular therapy; TASC type D lesions necessitate surgery, owing to endovascular therapy’s prohibitive failure rate; and TASC types B and C lesions can be treated using either endovascular or surgical revascularization, depending on the clinical scenario. Some evidence suggests that endovascular reconstruction may be beneficial in patients with extensive disease (e.g., TASC type C or D) who are facing imminent limb loss but are not candidates for surgical reconstruction.13–17 For a detailed description of the TASC classification system, see Chapter 109.
Infrapopliteal
Despite the presence of an infrapopliteal classification system in the original TASC guidelines, the revised TASC II did not delineate specific anatomic criteria for infrapopliteal endovascular therapy (see Table 114-1). This omission indicates a lack of consensus regarding the effectiveness of endovascular therapy for infrapopliteal arterial occlusive disease.12 The TASC II guidelines state that infrapopliteal angioplasty and stenting should be reserved for limb salvage therapy; there is insufficient evidence to recommend their use for the treatment of intermittent claudication. The only specific recommendation is that in a patient with CLI and medical comorbidities who is being treated for infrapopliteal occlusion, endovascular therapy may be attempted if inline flow to the foot can be reestablished. Therefore, as is the case with femoropopliteal disease, if a patient is at high risk for surgical bypass and is facing imminent limb loss, PTA may be considered to avoid amputation.18,19
Results
Reporting Standards
The revised Society for Vascular Surgery Lower Extremity Ischemia Reporting Standards (also known as the Rutherford guidelines) emphasize the need for uniform, objective criteria for evaluating the success of interventions for infrainguinal PAD.20 These guidelines recommend the use of specific clinical criteria or stages, as well as vascular laboratory data, to evaluate outcomes. With respect to chronic limb ischemia, affected limbs are categorized according to the following spectrum of symptoms: asymptomatic disease, mild claudication, moderate claudication, severe claudication, ischemic rest pain, minor tissue loss, and major tissue loss. Additional clinical and noninvasive hemodynamic criteria are used to quantitate changes in preprocedure and postprocedure clinical status (see Chapter 108).20 Although the revised Rutherford guidelines remain the standard for reporting on lower extremity PAD, they have been applied more consistently to reports of surgery than to those of endovascular therapy. Consequently, it may be useful to update and reenforce the use of standardized criteria for reporting the outcomes of lower extremity revascularization regardless of mode of intervention.21
Definitions of Successful Intervention
The complexity of infrainguinal endovascular therapy has resulted in the creation of multiple definitions of success. The measures of success reported most commonly in the literature include clinical response with regard to symptom resolution and limb salvage, technical success, primary patency, assisted primary patency, secondary patency, and target lesion revascularization. Depending on the study design and the intent of the investigators, significant variability exists in the use of these outcomes.
Clinical Response
The literature is characterized by significant heterogeneity in the reporting of clinical status for patients with infrainguinal PAD treated with endovascular therapy. The clinical variables that are evaluated most commonly and exhibit the greatest clinical relevance are resolution of symptoms, limb salvage, and patient survival.
Resolution of symptoms and limb salvage have been defined using various combinations of the following clinical criteria: sustained absence of symptoms or improvement by at least one (claudication) or two (tissue loss) categories according to the Rutherford classification; freedom from claudication among claudicants; 50% improvement in claudication distance; complete resolution of rest pain; relief of rest pain without the use of analgesics; resolution of claudication or rest pain and healing of ulcers with no or only minor amputations at 6 weeks; healing of ulcers; total ulcer healing; healing of minor amputations required for gangrene; freedom from areas of gangrene; absence of any requirement for revascularization; freedom from minor amputations; absence of amputation; standing and walking without a prosthesis; freedom from amputations proximal to the forefoot or ankle; health-related quality of life improvement; and freedom from any clinical deterioration. The most appropriate measure of clinical response according to the revised Rutherford guidelines combines clinical and noninvasive imaging data—for example, an increase of at least 1 point on the Rutherford scale concomitant with an ankle-brachial index (ABI) improvement of 0.15 or greater.20
Technical Success
The majority of studies have used angiographic criteria to define the technical success of infrainguinal endovascular therapy. Technical success is most often defined as the presence of antegrade flow through the treated lesion at the termination of the procedure.22–24 Certain studies have added further refinements and other requirements, such as the presence of less than 25% to 30% residual stenosis, lack of flow-limiting dissection by angiography at the termination of the procedure, flow to the pedal arch, and vascular laboratory studies demonstrating a duplex-derived peak systolic velocity ratio of 1.5 or less at the treatment site or an ABI improvement of 0.15 or greater.25–28 One study specifically defined technical success as good flow on angiography at the termination of the procedure and good flow on duplex ultrasonography before hospital discharge.29 Conversely, technical failure has been defined as failure to revascularize the target lesion.30
Hemodynamic Success
In addition to the concepts of clinical response and technical success, hemodynamic success is used to document the degree to which the procedure has improved limb blood flow. Hemodynamic success is defined as an increase in ABI of 0.10 to 0.15 or greater. Conversely, an ABI increase of less than 0.10 to 0.15 or a decrease in ABI is considered a hemodynamic failure.20 Another reported definition of hemodynamic success is the absence of an ABI decrease greater than 0.15 from the maximum early postprocedural level, or the demonstration of biphasic or triphasic waveforms with a peak systolic velocity less than 200 cm/sec or a peak systolic velocity ratio less than 2 at the site of the intervention.31 Using angiographic criteria, hemodynamic success has also been defined as the absence of occlusion or less than 30% to 50% residual stenosis in the treated vessel.32,33 According to the Rutherford guidelines, hemodynamic success must be accompanied by an improvement in clinical response to be designated a clinical success.20
Primary Patency
Primary patency has been interpreted in various ways in different studies. For arterial grafts, the definition of patency is clear, but in many cases of endovascular therapy, the artery was patent before treatment, so most investigators have included recurrent stenosis, as well as thrombosis, in the definition of patency after endovascular therapy. Primary patency is most frequently determined a study cohort by calculating the duration of follow-up in which there is an absence of occlusion or significant restenosis within the treated segment. For the determination of primary patency, the degree of allowable restenosis varies between 30% and 50%, depending on the cited literature.34–36 Other reported measures of primary patency have used data from noninvasive vascular studies to define significant recurrent stenosis: velocity ratio greater than 2.4 or ABI decrease greater than 0.15 results in loss of primary patency.23,26,37 Primary patency is typically reported in the context of a Kaplan-Meier life-table analysis.
Assisted Primary Patency
The definition of assisted primary patency is simply primary patency (as defined earlier) requiring the assistance of a subsequent interventional procedure to maintain patency or treat a significant recurrent stenosis. Typically, reintervention in nonoccluded but restenotic arteries carries the same or lower risks of morbidity and mortality as those associated with primary endovascular therapy.38 In addition, lower rates of primary patency have been observed for infrainguinal PAD compared with more proximal arterial vasculature disease. Therefore in conjunction with aggressive postprocedural monitoring, preocclusion reintervention may be required to prolong the duration of clinical patency in the cohort of patients undergoing infrainguinal endovascular therapy. Assisted primary patency is reported with Kaplan-Meier life-table analysis.
Secondary Patency
The definition of secondary patency differs from that of assisted primary patency in that it refers to patency that has been restored after occlusion of the treated arterial segment. Salvage of an occlusive lesion is currently achieved with safety and efficacy levels that approach those reported for reintervention of a preocclusive lesion. Therefore outcomes reported in terms of secondary patency are frequently similar to those using assisted primary patency. Secondary patency is also reported with Kaplan-Meier life-table analysis.
Target Lesion Revascularization
The concept of target lesion revascularization (TLR) was first applied to the percutaneous treatment of coronary artery disease to accurately identify the need to reintervene in a previously treated atherosclerotic lesion.39,40 TLR is typically defined as a reintervention performed on a restenosis at or within 5 mm of the lesion treated during the index procedure. In contrast, target vessel revascularization is defined as any repeat intervention, whether endovascular or open, that occurs in the same vessel treated during the index procedure. The concept of TLR is used to assess the safety and efficacy of percutaneous treatment, and the information it provides differs from that obtained from evaluating patency. TLR may, however, overestimate the benefit of an intervention because patients may elect not to undergo repeat intervention if their symptoms are mild, such as claudication. Thus significant restenosis could occur at the site of endovascular therapy for claudication, causing a return of symptoms but not result in TLR. For this reason, most regard TLR as a less accurate reporting method than the patency measures described earlier.
Determinants of Outcome
The primary determinants of outcome for patients undergoing infrainguinal endovascular therapy include lesion characteristics, pattern of vascular disease, patient demographics and comorbid diseases, clinical situation, and intraprocedural factors (Box 114-1). The majority of data can be gathered preoperatively during the history and physical examination and through the use of noninvasive testing. At the time of intervention, additional information with regard to lesion-specific characteristics may be obtained.
Lesion Characteristics
Location.
Endovascular therapy of proximal, larger-caliber arteries results in improved immediate and long-term outcomes compared with the treatment of distal, smaller-caliber arteries. Improved long-term patency has been reported for aortic and iliac artery lesions compared with infrainguinal lesions.41,42 For infrainguinal disease, preliminary results suggest a trend toward improved outcomes following the treatment of femoropopliteal lesions compared with infrapopliteal lesions.43,44 This trend is reflected in the revised TASC criteria, which delineate a broad range of indications for endovascular therapy of femoropopliteal lesions but continue to restrict infrapopliteal endovascular therapy to limb salvage therapy.12
Stenosis versus Occlusion.
Historically, endovascular therapy of stenoses yields better immediate and long-term outcomes than does the treatment of occlusions.45–47 Although more recent data indicate an improvement in the technical success rates associated with endovascular therapy of occlusions,1 long-term outcomes with regard to symptom resolution, limb salvage, and clinical patency continue to be superior for stenoses compared with occlusions. These differences may reflect the increased overall disease burden that accompanies occlusive lesions.8,46 Accordingly, the TASC criteria characterize occlusion as an anatomic factor that adversely affects outcome following endovascular therapy.12
Length.
The results of endovascular therapy of infrainguinal PAD are affected by lesion length. The length of the treated lesion is inversely correlated with successful outcome in terms of technical success, symptom resolution, limb salvage, and clinical patency.45,47,48 Early experience demonstrated that treated lesions shorter than 2 cm exhibited significantly greater patency than longer lesions. Five-year patency rates were approximately 75% for lesions less than 2 cm, versus 50% for longer lesions.48,49 Consequently, some investigators suggested that a long lesion should be considered a relative indication for stent placement. Outcomes following SIA, however, seemed to show a limited dependency on lesion length and more dependency on the presence of normal vessel above and below the lesion.25 These considerations are likewise reflected in the revised TASC criteria, which deemphasize lesion length as an adverse factor.12
Multiple Same-Segment Stenoses.
Endovascular therapy of multiple same-segment or multifocal stenoses results in worse outcomes compared with focal stenoses or lesions. The rationale is that multiple same-segment lesions represent multiple potential sites for immediate or long-term failure. Initially, 3-year patency rates of 20% following the treatment of long, multifocal stenoses were reported, compared with 68% following the treatment of focal stenoses.50 More recently, using a hybrid approach consisting of surgery and percutaneous techniques for the treatment of multifocal stenoses, 2-year primary and assisted primary patency rates were 79% and 86%, respectively.51
Pattern of Vascular Disease
Multilevel Disease.
Factors influencing the outcomes of endovascular therapy of multilevel disease are likely similar to those affecting multiple same-segment lesions. In multilevel disease, each lesion has its own failure rate and has the potential to impact the entire reconstruction. The outcome of multilevel disease is thought to be more favorable following surgery compared with endovascular therapy.12,52 In addition, patients with multilevel disease are frequently older, have multiple risk factors for atherosclerosis, and have lower baseline ABIs, putting them at increased risk for perioperative complications and long-term failure.53–55
Runoff Status.
Similar to the effect of outflow vessel quality on the success of open bypass, the success of infrainguinal endovascular therapy correlates directly with improved runoff status. In patients treated for femoropopliteal stenoses, the 5-year clinical success rate was 53% in patients with good runoff, compared with 31% in patients with poor runoff, in a representative study.46 The same study demonstrated that in patients treated for occlusions, the 5-year clinical success rate was 36% in patients with good runoff and 16% in patients with poor runoff. More recent data from a report evaluating 324 infrainguinal interventions confirmed the deleterious effect of poor runoff.38 One-year primary patency rates were significantly diminished in patients with impaired tibial runoff (fewer than three vessels) compared with patients having normal tibial runoff (three vessels)—52% versus 83%.
Patient Demographics
Gender.
Female gender is frequently cited as a factor that negatively influences the outcome of open or endovascular reconstruction. However, conflicting evidence exists concerning the patency and clinical success rates of infrainguinal intervention in women compared with men.30,56 One potential reason for these outcome differences is that the treated vessels, as well as the inflow and outflow vessels, are typically smaller in women. Even though female gender has been reported to be an independent risk factor for failure of lower extremity vascular reconstruction, these differences may be accounted for by the increased incidence of associated adverse factors in women, including CLI, diabetes, and infrapopliteal disease.57
Comorbidities.
Outcomes of infrainguinal endovascular therapy are worse in patients with diabetes, and the incidence of infrapopliteal disease is high in this patient subgroup. The interaction of these closely linked risk factors may explain the increased failure rates following endovascular therapy.58 After controlling for lesion location, however, the reported results are more variable. In some studies, diabetes does not independently predict poor outcome.59–61 Other reports suggest that type 1 and type 2 diabetes mellitus and hypertension are independent risk factors for loss of primary patency following endovascular therapy, and progression to CLI following endovascular therapy for claudication.44,62–64 End-stage renal disease may also decrease the success rate for endovascular infrainguinal therapy. A significant increase in SIA failure has been observed in patients with renal failure.65 In addition, diabetes and renal failure are found more commonly in patients presenting with CLI, which may also explain the diminished patency rates observed in these patient cohorts.66
Clinical Situation
Indication for Intervention.
The indication for intervention represents one of the strongest predictors of immediate and long-term outcome for patients undergoing infrainguinal endovascular therapy. With respect to lesion characteristics, multilevel disease, and comorbidities, patients with CLI present with more complex disease than those with claudication. Several studies have demonstrated that patients with claudication demonstrate better outcomes than those with CLI when evaluating symptom resolution and overall patency.10,46,48
Recurrent Stenosis.
Historically, the results of dilatation of recurrent infrainguinal stenoses were poor. Consequently, surgical reconstruction was recommended for recurrent stenoses. One study that characterized the early treatment experience of recurrent stenoses demonstrated that the 1-, 2-, and 3-year clinical success rates were 41%, 20%, and 11%, respectively, for endovascular therapy, compared with 84%, 72%, and 72%, respectively, for surgical reconstruction.67 Owing to improved techniques and devices, there is a trend to apply endovascular therapy more aggressively for recurrent stenoses.68 Definitive recommendations for the treatment of restenoses have not been made because the data are insufficient to assess effectiveness.
Intraprocedural Factors
Dissection or Residual Stenosis.
In addition to preprocedural factors that might affect the outcome of infrainguinal endovascular therapy, intraprocedural factors may play a role. Flow-limiting dissections and residual stenoses of 30% or greater occur with an estimated combined frequency of 10% with endovascular therapy.10,69 Traditionally, intraprocedural dissection or residual stenosis was a significant prognostic indicator of poor immediate and long-term outcome. With the advent of stent placement to prevent acute occlusion, early failure rates have improved significantly.70
Initial Hemodynamic Response.
Gauging immediate postprocedural anatomic success can be accomplished using digital subtraction angiography; however, ABI is also a significant prognostic indicator. An improvement in postprocedure ABI correlates with superior immediate and long-term outcome.20
Treatment Outcomes
Femoropopliteal: Angioplasty
Before the routine use of stenting, data for conventional femoropopliteal PTA revealed initial technical success rates of 80% to 95% (Fig. 114-1). However, long-term patency rates were significantly worse compared with those of patients treated for isolated aortoiliac disease. Reported primary patency rates were 47% to 63% (1 year), 38% to 51% (3 year), and 26% to 45% (5 year).45–47,71,72 A meta-analysis evaluated the long-term outcomes for femoropopliteal PTA from 19 studies conducted between 1993 and 2000.9 The study included 923 balloon dilatations and illustrated the significant impact of occlusive lesions and CLI on long-term outcome: 3-year primary patency rates were 61% for stenoses in claudicants, 48% for occlusions in claudicants, 43% for stenoses in patients with CLI, and 30% for occlusions in patients with CLI (Fig. 114-2).
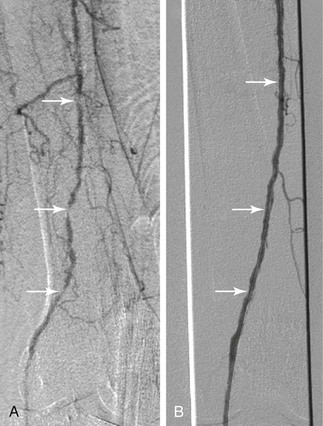
Figure 114-1 A 102-year-old woman with progressive cyanosis and early gangrene of the right great toe associated with ulceration. A, Arteriography demonstrates diffuse infrainguinal disease, including multiple same-segment stenoses and occlusions of the superficial femoral artery (arrows). B, The serial stenoses and occlusions were crossed using a glide wire. After 4-mm balloon angioplasty of the superficial femoral artery, angiography demonstrates resolution of the underlying stenoses and occlusions (arrows), with no evidence of significant residual stenosis or flow limitation. For this reason, no stents were placed.
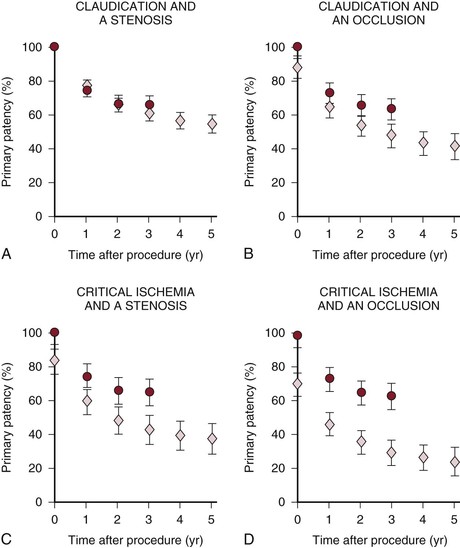
Figure 114-2 Cumulative primary patency rates and 95% confidence intervals (error bars) for femoropopliteal balloon dilatation (diamonds) and femoropopliteal stent implantation (circles), depending on lesion type (stenosis versus occlusion) and clinical indication (claudication versus critical ischemia). A, Graph shows that the estimates for primary patency following percutaneous transluminal angioplasty and stent placement are similar in patients with claudication and femoropopliteal stenosis. B through D, Graphs show that the estimates for primary patency following percutaneous transluminal angioplasty and stent placement are different in patients with critical ischemia and femoropopliteal occlusion. (From Muradin GS, et al: Balloon dilation and stent implantation for treatment of femoropopliteal arterial disease: meta-analysis. Radiology 221:137-145, 2001.)
SIA is a newer technique used to treat complex lesions and longer occlusions. The treatment of femoropopliteal artery occlusions using SIA was evaluated in 200 consecutive patients.73 Technical success was achieved in 80% of the patients treated and was not affected significantly by lesion length. In addition, hemodynamic success and clinical patency rates at 3 years were 46% and 48%, respectively. A recent systematic review evaluated studies from 1966 through May 2007 focusing specifically on infrainguinal SIA.8 A meta-analysis could not be performed because of the significant heterogeneity among the studies. The data incorporated 23 cohort studies and 1549 patients. Technical success ranged from 80% to 90%. One-year clinical success was achieved in 50% to 70% of patients, with limb salvage occurring in 80% to 90% of patients; primary patency approximated 50% in most series.
Although the use of angioplasty for complex femoropopliteal disease results in compromised durability, some evidence suggests that the transiently improved arterial circulation might confer a limb salvage benefit to patients who cannot undergo surgery due to prohibitive risk.15 An early retrospective evaluation of 97 treated limbs in 86 patients exhibiting end-stage occlusive disease in whom vascular reconstruction was not feasible demonstrated a technical success rate of 90%, 1-year primary patency of 43%, and overall limb salvage of 76%.74 A more recent study evaluating 50 patients with severe femoropopliteal disease demonstrated a technical success rate of 78% and a limb salvage rate of 42% at 2 years.13
Femoropopliteal: Angioplasty versus Bypass
The “gold standard” for comparing angioplasty to surgical bypass is the randomized control trial. Recruitment and randomization is inherently challenging in such trials in which the treatments exhibit marked differences. One of the initial trials of surgical revascularization versus PTA randomized 255 patients.75 Kaplan-Meier life-table analysis demonstrated no statistically significant difference in outcomes at 4 years. Nevertheless, patients treated with surgical revascularization demonstrated a trend toward improved primary patency but higher annual mortality rates, and patients treated with PTA exhibited a trend toward improved limb salvage. The Bypass versus Angioplasty in Severe Ischemia of the Leg (BASIL) trial was a multicenter randomized controlled trial that assigned 452 patients to a surgery-first or angioplasty-first approach.76 The primary endpoint was amputation-free survival, and at 6 months’ follow-up, no significant difference was observed in amputation-free survival between the two groups (Fig. 114-3). Health-related quality of life, as measured using the EuroQoL 5-D and short form 36, did not differ significantly between the two groups.
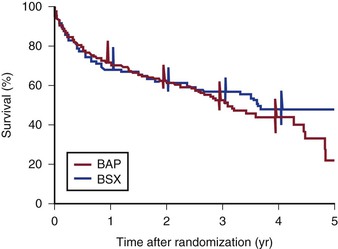
Figure 114-3 Amputation-free survival after bypass surgery (BSX) and balloon angioplasty (BAP). Bars show 95% confidence intervals for survival after 1, 2, 3, and 4 years of follow-up, which were calculated from the cumulative hazards. (From Adam DJ, et al: Bypass versus angioplasty in severe ischaemia of the leg [BASIL]: multicentre, randomised controlled trial. Lancet 366:1925-1934, 2005.)
Long-term follow-up for the BASIL trial was recently reported. In patients who survived at least 2 years, the surgery-first group had a mean improvement of 6 months for amputation-free survival with a hazard ratio of .85, and 7 months overall survival with a hazard ratio of .62.77 A post hoc on-treatment analysis of the BASIL trial revealed that patients who received angioplasty followed by bypass surgery fared worse than patients who received surgery alone in both amputation-free survival and overall survival.78 These findings contradict the widely held assumption that angioplasty can safely be performed as a temporizing measure before bypass and have subsequently been the subject of significant debate.
Despite mixed results for angioplasty in the BASIL trial, there has not been an appreciable reversion to open surgical bypass as a first-line therapy for infrainguinal occlusive disease. The minimally invasive nature of PTA combined with improvements in technology and operator experience have only served to reinforce a widespread trend towards an aggressive endovascular-first approach toward complex infrainguinal lesions.12,79
Femoropopliteal: Angioplasty versus Stenting
With the application of endovascular therapy to lesions of increasing complexity, stent placement has become integral to the management of infrainguinal disease (Fig. 114-4). Several trials have been published since the routine incorporation of stenting for infrainguinal PAD. Although the results have been inconsistent, stenting appears to be useful as a salvage procedure for suboptimal angioplasty. The initial experience from retrospective cohort studies demonstrated 1-year primary patency rates ranging from 49% to 81%.80–84 A meta-analysis evaluating 19 studies from 1993 to 2000, which included 473 stent placements, demonstrated 3-year patency rates ranging from 63% to 66%.9 Unlike the data for PTA, long-term patency following stent placement does not correlate with clinical indication or lesion type. Consequently, PTA and stenting appear to generate more favorable outcomes than PTA alone for severe femoropopliteal disease.
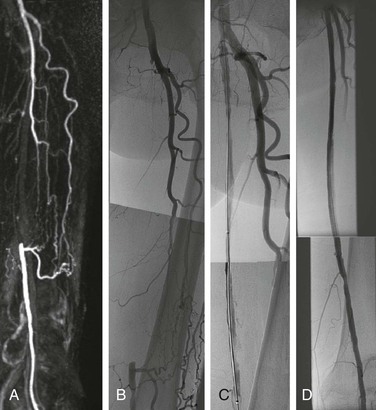
Figure 114-4 A, MRA showing a 20 cm-long segmental occlusion of the superficial femoral artery. B, Pretreatment angiogram. C, Angiogram after balloon angioplasty alone demonstrates flow limiting dissection and lesion recoil. D, Posttreatment angiogram after angioplasty and stent placement demonstrates resolution of all stenoses and dissections.
A number of randomized controlled trials have been designed to compare PTA alone with PTA and stenting. The following reports describe the results using balloon-expandable stents. Vroegindeweij et al randomized 51 patients to undergo either PTA or PTA with stenting.85 One-year clinical success, hemodynamic success, and primary patency did not differ significantly between the two groups. Cejna et al randomized 154 limbs to undergo either PTA alone or PTA with stenting.86 Although the rate of technical success was higher in patients undergoing PTA with stenting (99%, versus 84% with PTA alone), clinical success, hemodynamic success, primary patency, and secondary patency did not differ significantly between the two groups at 1 and 2 years after intervention. Becquemin et al randomized 227 patients with persistent disabling claudication or CLI to undergo either routine or selective stent placement using a balloon-expandable Palmaz stent.87 The primary endpoint evaluated was greater than 50% stenosis at 1-year follow-up, which did not differ significantly between the two groups. In addition, freedom from new vascular events and overall survival were not significantly different. These studies all indicate that the routine use of balloon-expandable stents for the treatment of femoropopliteal disease is not justified.
The lack of long-term efficacy associated with balloon-expandable stents for the treatment of femoropopliteal disease resulted in the pursuit of alternative stenting technologies, including the use of self-expanding nitinol stents. Conroy et al evaluated the results of treating 61 arteries in 48 men with chronic femoropopliteal occlusions, primarily using the Wallstent (Boston Scientific, Natick, Mass).88 The patients exhibited primary and secondary patency rates of 25% and 38%, respectively, at 4 years’ follow-up. Three retrospective studies were performed to evaluate the SMART stent (Cordis, Miami Lakes, Fla); they demonstrated 2-year primary patency rates of 60% to 84%.89–91
Several recent clinical trials have attempted to address the routine use of self-expandable stents in the superficial femoral artery (SFA), over PTA, and PTA with provisional stenting. In the ABSOLUTE trial, Schillinger and coworkers performed a randomized controlled trial of 154 patients with severe claudication or CLI due to superficial femoral artery (SFA) disease who underwent either angioplasty with optional stenting or angioplasty with routine stenting.92 The mean lesion length of patients undergoing PTA with provisional stenting was 9.2 cm versus 10.1 cm for patients undergoing primary stenting. Thirty-two percent of patients in the provisional stenting group underwent stent placement, most commonly owing to inadequate results following angioplasty. One-year restenosis rates differed significantly; they were 63% in the provisional stenting group and 37% in the routine stenting group (Fig. 114-5). In addition, patients treated with routine stenting were able to walk a greater distance before the recurrence of symptoms at 6 and 12 months compared with those treated with provisional stenting. In the RESILIENT trial, 206 patients with occlusive femoropopliteal and proximal popliteal lesions from multiple centers in the United States and Europe were randomized to receive either primary implantation of a self-expanding nitinol LifeStent (C.R. Bard, Murray Hill, NJ) or PTA with provisional stenting. The RESILENT trial had a slightly shorter mean lesion length than the Schillinger trial, with a mean of 7.1 cm for the PTA group and 6.4 cm for the primary stenting group. Patients in the primary stent group performed better at 12 months for the primary outcome, freedom from target lesion revascularization, at 87% compared to 45% for the PTA group. The primary stenting group also had better 12-month primary patency: 81% versus 37% with PTA alone.93 These encouraging results for primary stenting were not reproduced in the Femoral Artery Stenting trial (FAST), in which 244 patients with a single SFA lesion less than 10 cm were randomized to receive a self-expanding stent or PTA. In contrast to the ABSOLUTE and RESILIENT results, this trial had a significantly shorter mean lesion length of 4.5 cm and found no significant difference in the primary outcome—ultrasound-assessed restenosis at 1 year. Restenosis rates for PTA and stenting were 39% and 31% respectively. There was also no significant difference in Rutherford category improvement between the two groups.94 These results may be explained by the shorter lesion length which led to a higher rate of PTA failure in the FAST trial.
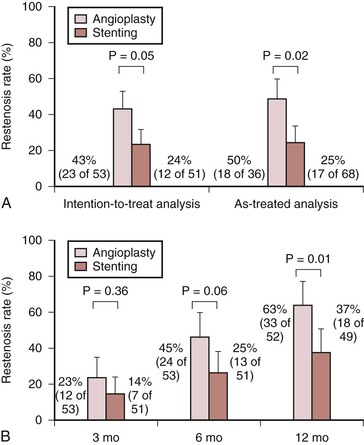
Figure 114-5 Rates of restenosis (defined as stenosis ≥50%). A, Rates of restenosis determined by angiography at 6 months, analyzed according to the intention-to-treat principle and according to the treatment actually received. Stenting demonstrated significantly diminished rates of restenosis compared with angioplasty when evaluated using angiography. B, Rates of restenosis in the same patients determined by duplex ultrasonography at 3, 6, and 12 months, analyzed according to the intention-to-treat principle. Error bars indicate 95% confidence intervals. At 6 and 12 months, stenting demonstrated significantly diminished rates of restenosis compared with angioplasty when evaluated using duplex ultrasonography. (From Schillinger M, et al: Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N Engl J Med 354:1879-1888, 2006.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree