Incidence of chronic heart failure (HF) increases with age and cardiovascular (CV) morbidity. Co-morbidities increase hospitalization and mortality in HF, and non-CV co-morbidities may lead to preventable hospitalizations. We studied the impact of co-morbidities on mortality and morbidity in Systolic Heart Failure Treatment with the I f Inhibitor Ivabradine Trial, and investigated whether the impact of ivabradine was affected by co-morbidities. We analyzed the Systolic Heart Failure Treatment with the I f Inhibitor Ivabradine Trialpopulation, with moderate-to-severe HF and left ventricular dysfunction (in sinus rhythm with heart rate at rest ≥70 beats/min), according to co-morbidity: chronic obstructive pulmonary disease, diabetes mellitus, anemia, stroke, impaired renal function, myocardial infarction, hypertension, and peripheral artery disease. Co-morbidity load was classed as 0, 1, 2, 3, 4+ or 1 to 2 co-morbidities, or 3+ co-morbidities. Co-morbidities were evenly distributed between the placebo and ivabradine groups. Patients with more co-morbidities were likely to be older, women, had more advanced HF, were less likely to be on β blockers, with an even distribution on ivabradine 2.5, 5, or 7.5 mg bid and placebo at all co-morbidity loads. Number of co-morbidities was related to outcomes. Cardiovascular death or HF hospitalization events significantly increased (p <0.0001) with co-morbidity load, with the most events in patients with >3 co-morbidities for both, ivabradine and placebo. There was no interaction between co-morbidity load and the treatment effects of ivabradine. Hospitalization rate was lower at all co-morbidity loads for ivabradine. In conclusion, cardiac and noncardiac co-morbidities significantly affect CV outcomes, particularly if there are >3 co-morbidities. The effect of heart rate reduction with ivabradine is maintained at all co-morbidity loads.
The prevalence and incidence of chronic heart failure (HF) steadily rises with increasing age and cardiovascular (CV) morbidity. The care of patients with HF becomes more complex because of aging-related cardiac and noncardiac co-morbidities, disabilities, and frailty. Moreover, treatment is progressively complicated by changes in drug pharmacokinetic and pharmacodynamics in the elderly, and with increased polypharmacologic therapy. This is associated with increased economic burden for health care providers. Co-morbidities increase hospitalization and mortality. It is unknown whether an increase in non-CV co-morbidities affects the efficacy of proved medications. The Systolic Heart Failure Treatment with the I f Inhibitor Ivabradine Trial (SHIFT) study tested the effect of heart rate reduction with the I f -inhibitor ivabradine in patients with systolic HF, left ventricular ejection fraction (LVEF) ≤35%, in sinus rhythm and with heart rate at rest ≥70 beats/min on maximized guidelines-directed background therapy. SHIFT showed a significant reduction of CV death and HF hospitalization with ivabradine-induced heart rate slowing. In the current post hoc analysis, we studied the impact of preexisting co-morbidities on mortality and morbidity in the SHIFT population and the treatment effect of ivabradine in the presence of co-morbidities.
Methods
The design and the main results of SHIFT have been published previously. In brief, SHIFT was a randomized, placebo-controlled, double-blind clinical trial in patients with moderate-to-severe HF and left ventricular dysfunction (LVEF ≤35%). Patients in sinus rhythm, aged >18 years with a heart rate at rest ≥70 beats/min at 2 consecutive visits, were randomized to either ivabradine or placebo. Ivabradine was started at 5 mg bid and adjusted when necessary, to either 7.5 mg or 2.5 mg bid depending on heart rate and tolerability. All SHIFT investigators were expected to include patients taking evidence-based medications at maximally tolerated doses for HF (including β blockers). When a patient was not on a β blocker, or not on evidence-based target doses of β blocker, investigators were required to explain why and record the reasons in a dedicated case report form. The primary end point was a composite of CV death or hospital admissions for worsening of HF. All the study end points were adjudicated by an independent end point validation committee. The 8 most prominent co-morbidities; chronic obstructive pulmonary disease (COPD), diabetes mellitus, anemia, stroke, impaired renal function (glomerular filtration rate ≤60 ml/min), myocardial infarction, hypertension and peripheral artery disease were reported in case report forms and evaluated. We studied the association of 1, 2, 3, and 4+ co-morbidities on CV and non-CV outcomes and to increase sample sizes in 0 co-morbidity, 1 to 2 co-morbidities, and 3 or more co-morbidities.
Descriptive statistics are presented as means and SD for continuous variables and as numbers and percentages for categorical variables. For baseline characteristics, the pooled placebo and ivabradine groups were divided into groups with different co-morbidity loads (1, 2, 3, 4+ or 0, 1 to 2, 3+ co-morbidities). Baseline characteristics were compared between the co-morbidity groupings using analysis of variance for continuous variables and a chi-square test for categorical variables. All time-to-event regression analyses were based on Cox proportional hazard models. Hazard ratios and 95% confidence intervals were estimated, and p values were calculated from the Wald statistic. Time-to-event curves for each treatment arm co-morbidity group were estimated using the Kaplan–Meier method. The effect of co-morbidity load on CV outcomes was assessed, in each treatment group separately, unadjusted and adjusted for beta-blocker use, New York Heart Association class, ventricular ejection fraction (LVEF), heart rate, ischemic or nonischemic pathology, age, and systolic blood pressure. The treatment effect of ivabradine versus placebo was assessed in the co-morbidity groups separately. We tested for evidence of a difference in the estimated treatment effect between the co-morbidity groups by adding a multiplicative interaction between treatment and co-morbidity group. Logistic regression analysis was used to assess each co-morbidity’s value as a predictor of heart rate (above/below 75 beats/min). The outcomes analyzed were the primary end point (composite of CV death or hospital admission for worsening of HF) as individual components as well as death from HF or all-cause death, CV hospitalizations, total hospitalizations, and non-CV hospitalizations. SAS (version 9.2) was used for all statistical analyses.
Results
Baseline characteristics of all patients and the impact of co-morbidities on baseline characteristics are depicted in Table 1 . Patients with higher numbers of co-morbidities and in particular with 3+ co-morbidities were likely to be older and more likely to be women with HF of ischemic origin. Furthermore, these patients also tended to have higher LVEF and more advanced clinical classes of HF. Patients with more co-morbidities were less likely to be on β blockers, but there were similar distributions of ivabradine and placebo between the different co-morbidity groups.
| Baseline characteristics | All patients (n=6505) | No comorbidities (n=685) | 1-2 comorbidities (n=3442) | 3+ comorbidities (n=2378) | P (trend) |
|---|---|---|---|---|---|
| COPD | 730 (11.2%) | 0 | 234 (6.8%) | 496 (20.9%) | <0.001 |
| Diabetes | 1979 (30.4%) | 0 | 587 (17.1%) | 1392 (58.5%) | <0.001 |
| Anemia < 120 | 492 (7.6%) | 0 | 123 (3.6%) | 369 (15.5%) | <0.001 |
| Stroke | 523 (8.0%) | 0 | 103 (3.0%) | 420 (17.7%) | <0.001 |
| EGFR < 60 | 1697 (26.1%) | 0 | 458 (13.3%) | 1239 (52.1%) | <0.001 |
| MI | 3666 (56.4%) | 0 | 1744 (50.7%) | 1922 (80.8%) | <0.001 |
| Hypertension | 4314 (66.3%) | 0 | 2150 (62.5%) | 2164 (91.0%) | <0.001 |
| PVD | 407 (6.3%) | 0 | 50 (1.5%) | 357 (15.0%) | <0.001 |
| EGFR | 74.6 (22.9) | 90.4 (22.1) | 78.7 (20.4) | 64.1 (22.1) | <0.001 |
| Age (years) | 60.9 (11.4) | 51.2 (12.7) | 59.9 (10.9) | 65.1 (9.4) | <0.001 |
| Male | 4970 (76.4%) | 546 (79.7%) | 2696 (78.3%) | 1728 (72.7%) | <0.001 |
| Ethnic origin | |||||
| White | 5771 (88.7%) | 505 (73.7%) | 3034 (88.1%) | 2232 (93.9%) | <0.001 |
| Asian | 532 (8.2%) | 148 (21.6%) | 278 (8.1%) | 106 (4.5%) | |
| Other | 202 (3.1%) | 32 (4.7%) | 130 (3.8%) | 40 (1.7%) | |
| Current smokers | 1118 (17.2%) | 122 (17.8%) | 648 (18.8%) | 348 (14.6%) | <0.001 |
| Body mass index (kg/m 2 ) mean (SD) | 28.0 (5.1) | 25.9 (5.0) | 27.9 (5.0) | 28.7 (5.0) | <0.001 |
| Resting heart rate (bpm) mean (SD) | 79.9 (9.6) | 81.8 (11.1) | 79.6 (9.4) | 79.7 (9.5) | <0.001 |
| Systolic blood pressure (mm Hg), mean (SD) | 121.7 (16.0) | 112.5 (14.1) | 121.5 (15.8) | 124.6 (15.6) | <0.001 |
| Diastolic blood pressure (mm Hg), mean (SD) | 75.7 (9.5) | 71.8 (8.7) | 76.0 (9.6) | 76.3 (9.3) | <0.001 |
| LVEF (%), mean (SD) | 29.0 (5.2) | 27.3 (5.7) | 29.0 (5.1) | 29.4 (5.0) | <0.001 |
| NYHA Class III/IV | 3334 (51.3%) | 292 (42.7%) | 1643 (47.7%) | 1399 (58.9%) | <0.001 |
| Ischemic heart failure | 4418 (67.9%) | 83 (12.1%) | 2243 (65.2%) | 2092 (88.0%) | <0.001 |
| History of atrial fibrillation or flutter | 522 (8.0%) | 33 (4.8%) | 249 (7.2%) | 240 (10.1%) | <0.001 |
| History of dyslipidaemia, | 1221 (18.8%) | 55 (8.0%) | 625 (18.2%) | 541 (22.8%) | <0.001 |
| ACE inhibitor ∗ | 5116 (78.6) | 527 (76.9%) | 2731 (79.3%) | 1858 (78.1%) | 0.981 |
| ARB ∗ | 927 (14.3%) | 82 (12.0%) | 470 (13.7%) | 375 (15.8%) | 0.004 |
| Diuretic ∗ | 5414 (83.2%) | 582 (85.0%) | 2771 (80.5%) | 2061 (86.7%) | <0.001 |
| Aldosterone ∗ , n | 3922 (60.3%) | 514 (75.0%) | 2049 (59.5%) | 1359 (57.1%) | <0.001 |
| Beta-blocker ∗ | 5820 (89.5%) | 620 (90.5%) | 3129 (90.9%) | 2071 (87.1%) | <0.001 |
| Ivabradine ∗ | 3241 (49.8%) | 325 (47.4%) | 1758 (51.1%) | 1158 (48.7%) | 0.682 |
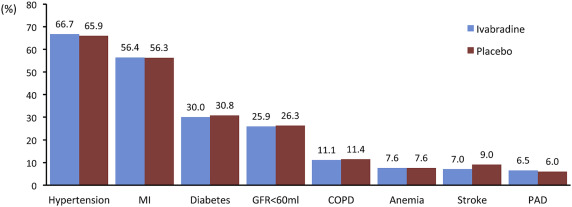
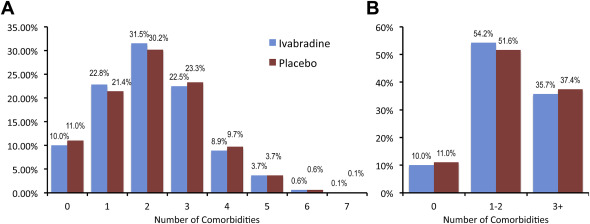
There was an even distribution of co-morbidities between placebo and ivabradine ( Figure 1 ). The most common co-morbidity was the history of hypertension, followed by myocardial infarction, diabetes and impaired renal function, COPD, anemia, stroke, and peripheral artery disease. Most patients had 1, 2, or 3 co-morbidities ( Figure 2 ), with 35.7% on ivabradine and 37.4% on placebo in patients with 3 or more co-morbidities ( Figure 2 ). Figure 3 shows Kaplan–Meier curves from the primary end point according to 0, 1, 2, 3, and 4+ co-morbidities on ivabradine and placebo. The numbers of CV death or HF hospitalizations were increased with cumulative co-morbidity load for both placebo and ivabradine. We grouped patients also for 0, 1 to 2, and 3 + co-morbidities with similar results. The event rates in each group tended to be lower with ivabradine compared with placebo in patients with 0 or 3+ co-morbidities. There was a highly significant association (p <0.0001) between co-morbidities and CV death and first HF hospitalization in both groups (not shown). Hospitalization rate was lower in all groups on ivabradine. Figure 4 shows the association of 0, 1, 2, 3, and 4+ co-morbidities on the primary end point, HF hospitalization, HF mortality, CV mortality, non-CV hospitalization, and total hospitalization on ivabradine and placebo. Co-morbidities were related to outcomes with 3 and for 4+ co-morbidities had constantly higher event rates. For most end points, estimated glomerular filtration rate was the strongest predictor. For all-cause hospitalization and non-CV hospitalization, COPD was most predictive.

In Supplementary Tables 1 to 4 , similar data of the effect of co-morbidities on the primary end point and its components as well as on HF mortality, all-cause mortality, CV hospitalization, total hospitalization and non-CV hospitalization are summarized. Mean heart rates at baseline did not show clinically meaningful differences significant across the different co-morbidity groups ( Table 1 ).
To study whether the co-morbidity load had an impact on the treatment effects of ivabradine, we investigated the hazard ratios for the primary and secondary end points. Figure 5 summarizes forest plots for the treatment effect of ivabradine versus placebo. Again, for all mortality and morbidity outcomes, there was a significant effect or a trend in favor of the superiority of ivabradine compared with placebo. No significant heterogeneity of the treatment effect of ivabradine was observed between the co-morbidity groups or different end points. In addition, there was no obvious effect of an increased load of co-morbidities on the size of the treatment effect of ivabradine ( Table 2 ). For all outcomes and for all groups of different co-morbidities, a trend without any evidence of heterogeneity was observed in favor of the treatment effect of ivabradine. Similar adjusted and nonadjusted patients were obtained when 0, 1, 2, 3, and 4+ groups were evaluated (Supplementary Tables 5 and 6 ).



