Environmental Disorders |
Both indoor and outdoor air pollution are of concern to pulmonary physicians. Exposures to indoor and outdoor air pollutants may both exacerbate and cause respiratory diseases and also increase the population’s risk for morbidity and mortality from malignant and nonmalignant diseases. This chapter provides a broad introduction to indoor and outdoor air pollution. It begins with a brief review of the emergence of indoor and outdoor air pollution as clinical and public health issues. The chapter then considers general principles and concepts related to inhalation injury, exposure, and health outcomes. The health consequences of indoor and outdoor air pollution are covered separately, although this distinction is artificial, given the penetration of outdoor pollutants into indoor environments and the overlap between the pollutants found in indoor and outdoor locations. The chapter concludes by considering two issues of direct concern to clinicians: susceptible populations and control strategies, both at the public health and individual levels. Pulmonary physicians have a key role in advising their patients and in carrying out research relevant to air quality management.
OVERVIEW
Air pollution has probably had adverse effects on health throughout history. The use of fire for heating and cooking brought exposure to smoke, a problem that persists today for the billions who still use biomass fuels, and the rise of cities concentrated the emissions of pollutants from dwellings and manufacturing facilities within restricted locales. Industrialization and electric power generation brought new point sources of pollution; that is, localized sources such as power plants, and sometimes immense emissions of combustion by-products, particles, nitrogen oxides (NOx), and sulfur oxides into areas where people lived and worked. During the twentieth century, mobile sources, including cars, trucks, and other fossil fuel–powered vehicles, created a new type of pollution – photochemical pollution, or “smog” – first recognized in the Los Angeles air basin in the 1940s. The unprecedented growth of some urban areas to form “megacities,” such as Mexico City, São Paulo, and Shanghai, has led to unrelenting air pollution from massive vehicle fleets, snarled traffic, and polluting industries and power plants. During the twentieth century, there was increasing recognition that the air pollution problem extends into indoor environments. In less-developed countries, exposure to smoke from biomass fuel combustion (e.g., open burning of wood for heating and cooking inside the home) is widespread, as it was in past centuries. In the more developed countries, indoor pollutants are generated by human activities (e.g., cooking, personal care products, etc.) and released from the materials used for construction and in furnishings, and often maintained at unhealthy concentrations by building designs that seal pollutants within.
Health effects of air pollution have long been of concern. During the reign of Edward I (1272–1307), the pollution of London by coal smoke prompted a royal proclamation banning burning of “sea coal” in open furnaces.1 In 1661, John Evelyn published Fumifugium or the Aer and Smoake of London Dissipated, describing an approach to the control of air pollution in London. However, air pollution was not regulated in England until approximately two centuries later with the passage of the Smoke Nuisance Abatement Act and the Alkali Act, directed at industrial pollution. In the United States, recognition of the public health dimensions of air pollution began in the middle of the twentieth century, driven by the rising problem of smog in southern California and the 1948 air pollution episode in Donora, Pennsylvania, which caused 20 excess deaths and thousands of illnesses.2 The first national legislation, the Air Pollution Control Act, was passed in the mid-1950s; the original Clean Air Act was passed in 19633 and most recently revised in 1990.4 It provides a comprehensive structure for air quality management in the United States.
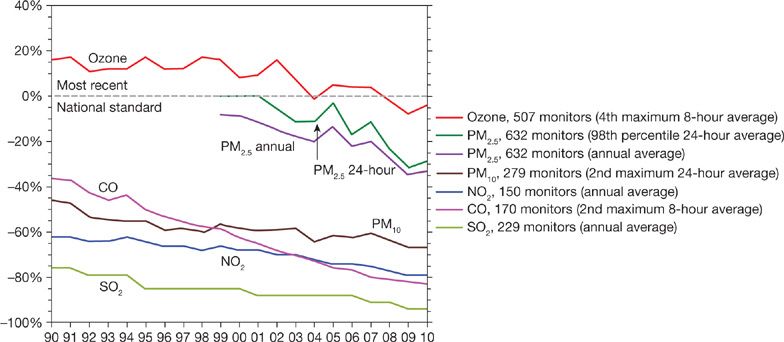
The modern era of air pollution research and control dates to the episodes in Donora and other cities, during which extremely high levels of pollution caused clearly evident excess deaths. The most dramatic episode was the London Fog of 1952, which caused thousands of excess deaths.5 These catastrophic episodes led to regulations for the control of outdoor air pollution and to the conduct of research designed to develop evidence on the health effects of outdoor air pollution as a foundation for control measures. The research included characterization of the pollutants in outdoor air as to their sources, concentrations, and chemical and physical properties; toxicological investigation on the injury caused by air pollutants and the underlying mechanisms; and epidemiological studies of the health effects of air pollution in the community. These approaches remain fundamental to research on air pollution. We now have a large body of evidence on the health effects of air pollution gained over more than a half-century of investigation and complex regulations that limit emissions and control concentrations of key pollutants in outdoor air. These evidence-driven regulations have had substantial impact on air quality in the United States, driving down levels of the most prominent pollutants (Fig. 91-1).6
Figure 91-1 Comparison of national levels of the six common pollutants to the most recent National Ambient Air Quality Standards, 1990–2010. National levels are averaged across all monitors with complete data for the time period.
In spite of these improvements in air quality, as indexed by specific pollutants, air pollution remains a significant public health concern. Epidemiological studies show that adverse health effects still occur at concentrations frequently observed and at the upper end of the range of the National Ambient Air Quality Standards (NAAQS). In addition, the approach of regulating concentrations of individual pollutants has left the problem of air pollution mixtures unsolved. One mixture of particular concern is that associated with high-density traffic.7 Studies conducted over the last two decades have linked this mixture to diverse adverse health effects, including lung cancer, allergies and asthma, respiratory symptoms, reduced lung function, and a variety of cardiovascular consequences. Global warming, a consequence of massive pollution of the atmosphere by “greenhouse” gases, has direct and indirect health consequences.8
The health effects of indoor air pollution are a more recent concern.9 Only limited measurements were made of indoor air contaminant levels before the 1970s, and the findings of the first large-scale studies of the health effects of indoor air pollution were not reported until the late 1960s and early 1970s. Pollutants of initial interest included secondhand smoke (SHS) or environmental tobacco smoke (ETS), the mixture of sidestream smoke and exhaled mainstream smoke inhaled involuntarily by nonsmokers, and nitrogen dioxide (NO2) generated by gas cooking stoves and ranges and by space heaters. Research soon broadened to biological agents, volatile organic compounds (VOCs), and two respiratory carcinogens—radon and asbestos (for further discussion on asbestos, see Chapter 86). Concern about the potential health effects of indoor air pollution was heightened by the design and construction of buildings with reduced exchange of indoor with outdoor air for the purpose of energy conservation; the reduction of air exchange was anticipated to diminish dilution and thereby increase indoor air pollutant concentrations. Beginning in the 1970s, outbreaks of nonspecific complaints started to occur among workers, who attributed their symptoms to the indoor environments in which they worked. Now referred to as sick building syndrome (SBS), these outbreaks continue but seemingly in smaller numbers than 10 years ago. Another syndrome, multiple chemical sensitivity, has also been linked to indoor air pollution; persons with this controversial syndrome, who may obtain consultation from pulmonary specialists, often report debilitating symptoms after exposure to indoor air contaminants, even at levels that may be considered generally safe. Another concern is disease resulting from potential exposure to mold in homes, particularly following water damage. The flooding of many homes in the Gulf States and New Jersey and New York by catastrophic hurricanes has served to reinforce the potential for chronic exposure to mold.
Control of indoor air pollution has been enacted primarily through nonregulatory approaches,9 as the Environmental Protection Agency (EPA) does not directly regulate the levels of pollutants in indoor air. The cornerstone of the control of indoor air pollution has been education of the public, manufacturers, and employers on approaches for reducing exposures and for reducing emissions from indoor sources. The EPA has given a guideline value for an acceptable indoor radon concentration; it has also proposed that all homes be tested for radon and the homes modified if the concentration is above the guideline.10 The handling of asbestos in schools was regulated under the Asbestos Hazard Emergency Reduction Act, and the agency has classified ETS as a class A carcinogen. A rising number of states and municipalities have banned smoking in public places and workplaces. For the majority of households with one or more smokers, some sort of policy is in place to address smoking, either by limiting it to specific locations or not allowing it indoors.
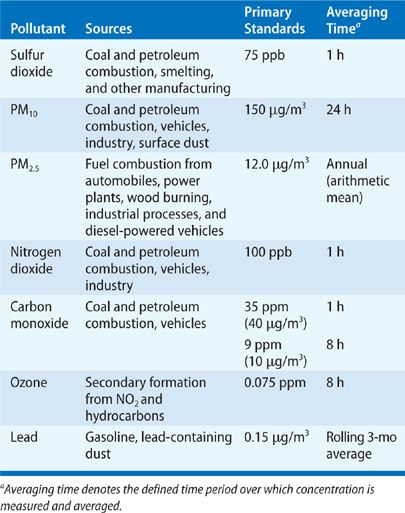
The literature on air pollution is now voluminous and has been published in a broad array of journals and technical reports. Of necessity, this chapter is selective in its review; emphasis has been placed on the most relevant findings for clinicians and on the newer literature. The documents prepared by the EPA on the six “criteria” pollutants (sulfur dioxide [SO2], particulate matter, NO2, carbon monoxide [CO], ozone [O3], and lead) offer encyclopedic reviews that are updated periodically (Table 91-1). Key documents on specific pollutants are cited within the appropriate sections of this chapter.
GENERAL PRINCIPLES AND CONCEPTS
Adverse responses to air pollutants reflect exposure and the delivery of the dose of the injurious agent to the target site within the respiratory tract. Air pollutants cause disease through various mechanisms. This section of the chapter covers principles of inhalation injury and the related spectrum of adverse health effects; it also covers principles of exposure assessment. The research methods used to characterize the effects of air pollutants are also detailed.
 PRINCIPLES OF INHALATION INJURY
PRINCIPLES OF INHALATION INJURY
Atmospheric pollutants, whether indoors or outdoors, exist in both gaseous and particulate forms. In evaluating clinical consequences of specific exposures, the clinician should recognize that penetration into and retention within the respiratory tract of toxic gases can vary widely, depending on the physical properties of the gas (e.g., solubility), the concentration of the gas in the inspired air, the rate and depth of ventilation, and the extent to which the material is reactive. Gases that are highly water soluble, such as SO2, are almost completely extracted by the upper airways of healthy subjects during brief exposures at rest. In contrast, removal of less water-soluble gases, such as NO2 or O3, is much less complete, and these gases may penetrate to the airways and alveoli of the respiratory tract. CO is poorly soluble in water and is not removed in the upper airways. On reaching the lung, CO diffuses across the alveolar–capillary membrane and then binds avidly to hemoglobin.
Exercise greatly augments penetration of gases into the deep lung and, thus, the total dose of pollutants delivered to targets in the airways. Exercise increases the dose directly by increasing minute ventilation; also, because many people switch from the nasal to the oral breathing route during moderate to heavy exercise, the more efficient pollutant removal in the nasal passages is replaced by the less efficient removal in the oral airway.
Particulate pollutants usually occur in nature as aerosols. Small liquid droplets or solid particles are dispersed in the atmosphere with sufficient stability to remain in an aerosol suspension. Examples of common aerosols are sulfuric acid mists and sulfate and nitrate salts formed from SO2 and NO2, respectively. Deposition of inhaled particles depends on many factors, including the aerodynamic properties of the particle (primarily size), airway anatomy, and breathing pattern. Particles greater than 10 μm are effectively filtered out in the nose and nasopharynx, where these relatively large particles are deposited efficiently because of impaction against surfaces and gravitational forces. Particles trapped in the nose and nasopharynx are cleared in secretions and coughed out or swallowed. Particles less than 10 μm in aerodynamic diameter (PM10) may be deposited in the tracheobronchial tree; deposition in the lung’s alveoli is maximal for particles less than 1 to 2 μm in diameter. Particles less than 0.5 μm move by diffusion to the alveolar level, where they collide with gas molecules by brownian movement and are impacted on alveolar surfaces. Recent interest has focused on both environmental and artificial particles in the so-called “ultrafine” range, that is, particles less than 100 nm in size; despite their extremely small size, high deposition has been found in the respiratory tract and total deposition increases as the particles get smaller. Removal of particles from the larger airways by the mucociliary apparatus is efficient and occurs within hours of deposition; clearance from the deep lung by alveolar macrophages is much slower, requiring days to months. Although particulate matter toxicity has been primarily linked with fine particles, recent studies have indicated that both the ultrafine11 and the coarse fractions12 are associated with adverse cardiopulmonary effects.
The mechanisms by which inhaled gases and particles injure the lung are diverse and not yet fully understood. Oxidant gases, O3 and NO2, cause inflammation of the respiratory epithelium, presumably through the production of toxic oxidant species and release of potent mediators. SO2 is also an irritant gas. The response to particles likely depends on the chemical and physical nature of the particles. Oxidizing compounds on particles may dissolve into tissue fluids and induce an inflammatory response. Organic materials on particles may also produce inflammation or act as initiators or promoters of cancer. Particles, once thought to only adversely affect the lungs, may also trigger acute cardiovascular events, in part through effects on pulmonary inflammation and on pulmonary reflexes, via oxidative stress, endothelial dysfunction, autonomic dysfunction, platelet activation, coagulation, etc. A recent review by the American Heart Association discusses these mechanisms and studies of air pollution effects on them in greater detail.13 The respiratory track, of course, is exposed to multiple pollutants, and interactions among them may synergistically increase effects. Exposure to oxidant gases, for example, enhances responses to inhaled allergens.
 ADVERSE HEALTH EFFECTS OF AIR POLLUTION: CLINICAL AND PUBLIC HEALTH CONCERNS
ADVERSE HEALTH EFFECTS OF AIR POLLUTION: CLINICAL AND PUBLIC HEALTH CONCERNS
The spectrum of adverse effects of air pollution is broad, ranging from the consequences of acute and dramatic exposures, which may cause death, to far more subtle and chronic effects on disease risk and well-being. This spectrum has been conceptualized as a pyramid with mortality at its tip and an increasingly common set of morbidities as the base. Perhaps the most common “adverse” effect is a loss of well-being from the diminished aesthetic value of a polluted environment. Clinicians are more likely to be concerned with the less common, more acute effects with clinical consequences—acute responses, often in asthmatics, for which a link to air pollution exposure may be made by history or challenge testing; the more subtle and long-term consequences are typically a focus for public health researchers and regulators. Nevertheless, clinicians may be asked to assess risks of long-term exposures or to estimate the contribution of exposures to disease causation in a particular patient. They may also be asked to guide their communities in evaluating air pollution as a local public health problem.
To interpret the scientific evidence on the effects of air pollution, clinicians need a framework for determining whether an effect is “adverse.” Judgment on the adversity of responses is societal and reflective of prevalent valuations and perceptions of risk. The Clean Air Act uses the term “adverse” without definition. If a broad construct of health is used that includes a state of well-being as a component, adverse effects of air pollution include not only clinically evident disease but also more subtle symptom responses and physiological effects that may compromise well-being or increase the risk of disease. In a report issued in 2000, a committee of the American Thoracic Society (ATS) offered guidelines for defining adverse respiratory health effects in epidemiological studies of outdoor air pollution.14 The committee turned to a medical basis for this determination, defining adverse respiratory health effects as medically significant physiological or pathological changes. Increasingly, research focuses on preclinical markers and whether acute changes in panels of markers constitute an adverse health impact remain uncertain.
Indoor air pollution has a broad range of effects as well (Table 91-2).15 Cases of clinically evident disease caused by indoor air pollution occur, and an unquestionable causal link can often be established for specific persons from a careful history or appropriate diagnostic testing, as with hypersensitivity pneumonitis. Indoor air pollution can also exacerbate chronic respiratory diseases—for example, house dust mite antigen and asthma in house dust mite-allergic persons. More subtle effects have become an increasing concern as we have learned that indoor air pollution can adversely affect comfort and increase risk for future disease; consequently, even the perception of exposure to indoor pollutants may adversely affect well-being. Radon and asbestos, for example, are respiratory carcinogens, which are presumed to increase risk of lung cancer.
Source: Data from Samet JM. Indoor air pollution: A public health perspective. Indoor Air. 1993;3:219–226.
 CONCEPTS OF TIME–ACTIVITY AND TOTAL PERSONAL EXPOSURE
CONCEPTS OF TIME–ACTIVITY AND TOTAL PERSONAL EXPOSURE
Definitions of concentration, exposure, and dose are fundamental to considering the health effects of air pollution and steps to reduce risk.16 Concentration refers to the amount of material present in air. Exposure constitutes contact with a material at a portal of entry into the body—the respiratory tract, gastrointestinal tract, and skin. For the lung, exposure would constitute the time spent in contaminated air. Exposure is calculated as the unit of concentration multiplied by time. Dose refers to the amount of material that enters the body; biologically effective dose is the amount of material reaching target sites for injury—for example, the mass of respirable particles delivered to the small airways. For example, the concentration of particles less than 10 μm in aerodynamic diameter (PM10) might be 100 μg/m3. A person spending 10 hours at this concentration would have an exposure of 10-hour times 100 μg/m3, or 1000 μg/m3 × h. Assuming lung deposition to be 50% of the total mass and a minute volume of 10 L/min, the deposited dose of PM10 would be 300 μg (0.5 × 100 μg/m3 × 10 L/min × 600 min × 0.001 L/m3). For most inhaled pollutants, dose will vary with activity level which drives ventilation rate.
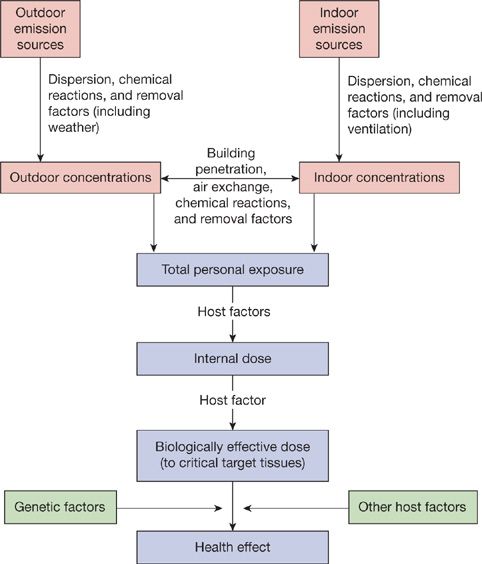
With regard to impact on health, total personal exposure to a pollutant is the relevant index of exposure, not the exposures received separately within indoor and outdoor environments. The total personal exposure of a person to a pollutant can be conceptualized as the time-weighted average pollutant concentration in the “microenvironments” in which the person spends time (Fig. 91-2).17 The microenvironments are locations having relatively constant concentration of the pollutant during the time spent there. The principal microenvironments contributing to total personal exposure are those with relatively high concentrations or in which relatively large amounts of time are spent. For example, for exposure to particles, key microenvironments might include an office in which smoking is allowed and an urban environment in which a home is located and time is spent outdoors and indoors. New sensing approaches are facilitating more refined studies of activity levels and air pollution and will sharpen understanding of where and when the most risky exposures take place.
Figure 91-2 Framework for conceptualizing exposure, dose, and health effects from outdoor and indoor air pollution. (Based on National Research Council data.)
Studies of time–activity patterns indicate that residents of more developed countries spend most of their time indoors and, consequently, personal exposures to many pollutants take place largely in indoor microenvironments. However, pollutants generated by outdoor sources do penetrate indoors, so indoor microenvironments can contribute to exposures to pollutants typically considered outdoor pollutants, such as particles and CO. Data on time use in a number of countries showed that people spend an average of 65% to 75% of their time inside their residences and more than 90% of time indoors, counting time at home, work, and elsewhere. Data from a 1987 to 1988 survey of Californians show a similar pattern, with employed adults averaging 15 hours per day indoors at home and 6 hours per day in other indoor settings. In the California study, school-age children spent an average of 18 hours indoors at home.18 National data from 1992 to 1994 were very similar.19 While these data emphasize the predominance of indoor microenvironments in determining exposures to many pollutants, exposure outdoors may be the predominant determinant of dose for some pollutants. For example, the dose of O3 (which generally has low indoor levels) received by the lung’s airways may be dominated by exposure received outdoors, particularly for people exercising or working outdoors.
 RESEARCH APPROACHES TO AIR POLLUTION
RESEARCH APPROACHES TO AIR POLLUTION
Our understanding of the health effects of air pollution derives from a tripartite research approach: characterization of atmospheric pollutants and exposures, toxicological studies, and epidemiological studies. These approaches are complementary. There has long been research on the physical and chemical properties of air pollutants, and more recently, exposure assessment has emerged as a separate research discipline. The tools of the exposure assessor include questionnaires that capture activities and time use, personal and area monitoring of air pollution levels, statistical models using these data to estimate exposures, and increasingly sophisticated biomarkers of exposure and dose.16 There is anticipation that new high-throughput technologies will facilitate data-rich measurement of “the exposome,” a variety of biomarkers that will complement the existing approaches.
Toxicological studies are often conducted to characterize the hazards of air pollutants; research may entail exposures of animals to one or more pollutants to assess patterns of injury and disease risk. Increasingly, toxicological approaches are used to characterize the relationship between exposure and dose and the mechanisms underlying injury. This mechanistic information addresses the appropriateness of extrapolating from animal studies to humans, particularly if parallel data from humans are available on dosimetry and mechanisms. Toxicological studies in which volunteers are exposed to pollutants in a controlled setting (e.g., exposure chamber or exposure room sealed off), often referred to as clinical studies, have proved to be an informative approach for investigating the acute consequences of pollution exposure. Such studies have been carried out using gases, for example, NO2 and SO2, and particles, including concentrated ambient particles and ultrafine particles (UFPs). In addition to healthy volunteers, groups in the population considered susceptible to the effects of the pollutant(s) of concern may be selected for investigation—for example, persons with asthma, chronic obstructive pulmonary disease (COPD), or coronary artery disease. Of necessity, exposures in clinical studies are of brief duration and ethically limited to levels that will have limited, transient effects. In addition to monitoring symptoms and physiological measures, clinical studies may be strengthened by a more invasive collection of biological specimens, using phlebotomy, nasal lavage, or fiberoptic bronchoscopy with biopsy of the mucosa or bronchoalveolar lavage (BAL), to elucidate mechanisms of injury. Molecular approaches using microarrays to analyze changes in gene expression are now being applied to cells (e.g., macrophages and blood monocytes) removed from humans following controlled exposure to pollutants. Clinical studies are also incorporating analyses to identify genetic polymorphisms that determine susceptibility to air pollutants.
There is also growing expectation for using high-throughput testing for assessing the toxicity of various chemicals including air pollutants. Various types of assays are being developed for this purpose; such testing is considered the cornerstone for future predictive toxicology. These approaches envision increased efficiency in toxicity testing and decreased animal usage by transitioning from lengthy in vivo testing to in vitro toxicity assays on human cells or cell lines with mechanistic quantitative parameters. Risk assessment in the exposed human population would focus on avoiding significant perturbations in these toxicity pathways.20
Epidemiological studies provide an assessment of the adverse effects of pollution exposures under the circumstances of “real world” exposure.17 The principal epidemiological study designs used for air pollution research include the following: cross-sectional studies or surveys; short-term cohort studies with intensive measurements of exposures and outcomes (i.e., panel studies); longer-term cohort studies directed at mortality and chronic disease risk21; time-series studies that assess short-term changes in outcomes (e.g., daily mortality counts) in relation to changes in daily air pollution levels22; and case-crossover studies that contrast the pollution exposure for an individual subject right before he or she had a health event to air pollution exposures of that same subject at other times when that person did not have that health event.23 There have been several landmark cohort studies, such as the Six Cities Study24 and the Children’s Health Study,25 but few studies of this design are likely to be implemented in the future, at least in countries like the United States where air pollution levels have declined substantially in recent decades. In part because of feasibility, time-series studies of morbidity and mortality are the most frequent.
The findings of epidemiological studies of air pollution have direct public health and regulatory relevance. The exposures are inherently representative of those received in the community, and the pollutants are inhaled in the form of the complex mixtures that actually exist in indoor and outdoor air. In addition, the community members in a study can be selected from the full spectrum of potentially susceptible subjects. There are, however, weaknesses to the epidemiological approach. Exposures to pollutants may be difficult to measure accurately, particularly past exposures that are used to study current health status. Hence, exposure estimates in epidemiological studies are subject to substantial error, both random and systematic. Generally such errors are likely to lead to underestimation of risk but overestimation is also possible. The effects of other exposures that are correlated with the air pollution exposure of interest and are themselves a predictor of the health outcome of interest, termed confounding factors, may not be sufficiently controlled, and thus when not accounted for in the analysis, may artefactually increase or decrease the apparent effect of air pollution exposure. Epidemiological studies having inadequate sample size and, therefore, limited statistical power may supply imprecise and uninformative estimates of risk and not precisely answer public health questions.
The technique of quantitative risk assessment has been increasingly applied to estimate the burden of disease associated with air pollution, particularly carcinogens.26 The 1990 Clean Air Act amendments include specific provisions on the use of risk assessment, particularly in regard to the hazardous air pollutants regulated under Section 112 of the Act. This process integrates the information on exposure and dose response to provide a characterization of the impact of an environmental agent on the population’s health; as the evidence is systematically reviewed in the conduct of a risk assessment, gaps in the evidence and attendant uncertainties are identified, and assumptions are made to fill the gaps.26 The approach was also used by the World Health Organization in its Global Burden of Disease estimates, which covered indoor and outdoor air pollution.
Risk assessment can be conceptualized as comprising the four steps outlined in the seminal 1983 report of the National Research Council (Table 91-3).27 A full risk assessment can be a large undertaking, requiring review of all relevant data and mathematical modeling to characterize the risk. It should be undertaken with a clear framing of the questions to be addressed. In a risk assessment, gaps in the scientific evidence, which are sources of uncertainty, are catalogued and used to estimate the level of confidence to attach to the risk characterization. The findings of risk assessment guide risk management, the process by which decisions are made about the need for risk reduction and the approaches to be implemented to reduce risks. Risk management means choosing among the options to control risk and balancing risk reduction, costs, and technological capability for reducing exposure. Uncertainties in the scientific information that have been catalogued in the risk assessment process may cloud risk management and introduce ambiguity regarding the optimum strategy. Nevertheless, risk managers need to make decisions in the face of uncertainty.
Source: Data from National Research Council (NRC), Committee on the Institutional Means for Assessment of Risks to Public Health. Risk Assessment in the Federal Government: Managing the Process. Washington, DC: National Academy Press; 1983.
Understanding the effects of complex mixtures of pollutants in indoor and outdoor air has proved particularly daunting for researchers. Exposures to pollutants rarely occur singly, without simultaneous exposures to other pollutants in the relevant microenvironments. Many outdoor sources inherently produce complex pollutant mixtures; for example, power plants release particles, NOx, and sulfur oxides, and vehicle exhaust contains CO, NOx, particles, and hydrocarbons. Indoor air is inevitably contaminated by complex mixtures, reflecting the multiplicity of sources in indoor environments. Synergistic or antagonistic interactions between components of complex pollutant mixtures could produce unanticipated effects, based on the toxicity of individual components.
OUTDOOR AIR POLLUTION
Outdoor air pollutants have diverse sources, both artificial and natural. This section begins with a review of the sources and then considers the effects of the principal artificial outdoor pollutants. The pollutants are grouped according to their designation by the EPA.
 OVERVIEW: SOURCES AND CLASSIFICATION OF OUTDOOR AIR POLLUTION
OVERVIEW: SOURCES AND CLASSIFICATION OF OUTDOOR AIR POLLUTION
Many pollutants, from both artificial and natural sources, can be found in outdoor air. Some naturally occurring pollutants in outdoor air are well documented as causing or exacerbating pulmonary diseases—for example, pollens and fungi. This chapter does not address these biological agents but considers the artificial pollutants. Researchers have focused more attention on the effects of artificial pollutants, which may reach potentially hazardous levels in urban areas or near-point sources, such as power plants, smelters, or manufacturing facilities. In the United States, the principal outdoor pollutants are generally classified within the framework provided by the Clean Air Act, which identifies two sets of air pollutants, “criteria” pollutants (Table 91-1) and “toxic” air pollutants. The term criteria refers to the standard-setting process for these pollutants, which requires preparation of a criteria document reviewing all relevant evidence every 5 years. The criteria pollutants include primarily combustion-related pollutants (SO2, NO2, CO, and particles), the secondary pollutant O3, and lead. The toxic pollutants are predominantly carcinogens, such as asbestos and radionuclides, and irritants. The sources are diverse but principally comprise industrial emissions and waste products. Examples of these pollutants are benzene, chlordane, ethylene oxide, hydrochloric acid, methane, parathion, propylene oxide, toluene, and vinyl chloride.
These two groups of pollutants are regulated through different mechanisms. For the criteria pollutants, NAAQS are set after extensive review of all relevant evidence. The standards must afford protection to the entire population, including those with heightened susceptibility, and offer an “adequate margin of safety.” For the hazardous pollutants, standards for maximum concentrations are intended to provide a margin of safety. The Clean Air Act includes mechanisms for achieving levels within the standards and enforcement. In spite of existing federal standards for ambient air quality, excesses are common in many areas of the country, most often for O3. Although the pollutants are considered in the following section on an individual basis, it should be re-emphasized that exposures of the population occur most often to mixtures, and synergism among individual pollutants may increase the effects of the mixture beyond the expected effect based on the components.
 OUTDOOR AIR POLLUTANTS: EXPOSURES AND HEALTH EFFECTS
OUTDOOR AIR POLLUTANTS: EXPOSURES AND HEALTH EFFECTS
The pollutants covered in this section are of public health significance throughout the world. Sulfur oxides, particles, NOx, and CO are generated by combustion and are typically found together in the complex air pollutant mixtures in outdoor environments. O3 is a secondary pollutant. While the pollutants are considered individually, exposures to them typically occur in the form of inhaled mixtures.
Sulfur Dioxide
Sulfur oxides are produced by combustion of fuels containing sulfur, such as coal from the Eastern United States and crude petroleum. Because of changing patterns of fuel use, reduction of acid emissions to control acid rain, and various other control strategies, pollution with sulfur oxides is far less prevalent nationally, while remaining important in some locales with point sources. Smelting of ores containing sulfur is also a prominent source in some regions, such as the southwestern United States. In the past, scientific research and regulatory concern in relation to the sulfur oxides were directed primarily at the health effects of SO2, the criteria pollutant regulated by the EPA. SO2 is a water-soluble gas that is effectively scrubbed from inspired air by the upper airway. Exercise, however, may increase the inhaled dose by its effect on minute ventilation and the switch to the oral breathing route. This pollutant has been shown to have adverse effects without concomitant exposures to other pollutants.28,29 In fact, exposures of volunteers to SO2 alone show that the gas may have adverse respiratory effects. Asthmatics are particularly sensitive, with some asthmatics having more severe health responses at a particular concentration than others with asthma (see below for specific examples). Significant exposures to SO2 alone might result from plumes released by smelters processing sulfur-containing ores or from other industrial processes.
Exposures to SO2 in outdoor air occur primarily with simultaneous exposures to other combustion-related pollutants, including NOx and particles. Heavy industry and coal-burning power plants are predominant sources for this type of pollutant mixture. Tall smokestacks for power plants, used to control local pollutant concentrations, release sulfur oxides and NOx high into the atmosphere, where residence time is prolonged. Through a series of chemical reactions, the sulfur oxides and NOx form acidic sulfate and nitrate particles, which may undergo long-range transport.30 These acidic particles represent a regional air pollution problem—blanketing, for example, the Central and Northeastern United States and portions of Canada. Fortunately, concentrations of SO2 have fallen in the United States, in part due to controls implemented under the Environmental Protection Agency’s Acid Rain Program, and from changing patterns of fuel use and energy generation. From 1983 to 2002, the average annual concentration fell about 50%. The effects of particulate air pollution and acidic aerosols are considered separately below. This section considers the effects of gaseous SO2.
Asthmatics are particularly susceptible to SO2, responding to exposure in chambers with increased airway resistance and reduced levels of lung function. With exercise and hyperventilation, which increase the dose delivered to the respiratory tract, some asthmatics are adversely affected at levels common in ambient air and well below those that might occur transiently with direct exposure to the plume from a power plant or factory. Inhalation of SO2 produces an immediate response within minutes and does not provoke delayed reactions several hours later or repetitive nocturnal attacks. The decrements in lung function on breathing of SO2 may be sufficient to produce symptoms of dyspnea, wheezing, and chest tightness. The bronchoconstriction resolves within an hour of exposure, and peak bronchoconstrictor responses may lessen on repeated challenge after a short recovery period. Responses to SO2 can be partly blocked by pretreatment with cromolyn sodium and anticholinergics and can be reversed by β-adrenergic agonists. Sequential exposures to SO2 and oxidant gases (O3 and NO2) have been performed in asthmatics; these clinical studies have provided little evidence of synergistic interactions in reducing airway function. Although some asthmatics have been shown to be affected by SO2 with exposure at low concentrations in the laboratory,31 complementary epidemiological data have not been reported that document parallel community morbidity.
Epidemiological studies from Hong Kong have examined the consequences of a major reduction in sulfur content in fuels over a very short period of time.32 The investigators found an associated substantial reduction in health effects (childhood respiratory disease and all age mortality outcomes). Daily SO2 was significantly associated with daily mortality in 12 Canadian cities with an average concentration of only 5 μg/m3.33 Nevertheless, there is still considerable uncertainty as to whether SO2 is the pollutant responsible for the observed adverse effects or, rather, a surrogate indicator for UFPs or some other correlated substance. For example, in Germany34 and the Netherlands35 a strong reduction of SO2 concentrations occurred over a decade. Although mortality decreased with time, the association of SO2 and mortality was judged as noncausal and attributed to a similar time trend of particulate matter. Wichmann et al. 34 in Germany considered the persistence of the SO2 effect as artifact because the SO2 concentration was much below levels at which effects were usually expected. Furthermore, the results for SO2 were inconsistent with those from earlier studies conducted in Erfurt. They concluded that both fine particles (represented by particle mass) and UFPs (represented by particle number) showed independent effects on mortality at ambient concentrations. Comparable associations for gaseous pollutants were interpreted as artifacts of collinearity with particles from the same sources.
Nitrogen Dioxide
NOx, like sulfur oxides, are produced by combustion processes (i.e., primary pollutants) and contribute to the formation of aerosols.28–30 Even though NO2 is regulated by the EPA as a criteria pollutant, substantial personal exposure in the United States occurs in indoor microenvironments contaminated by unvented gas stoves and space heaters. The principal source of NO2 in outdoor air is motor vehicle emissions, but power plants and industrial sources may also contribute. There have been few locations where point sources were strong enough to make NO2 alone a source of concern. The health effects of NO2 released into outdoor air probably arise principally from the formation of secondary pollutants. NO2 is an essential precursor of O3, and one of the principal pathways by which NO2 in outdoor air adversely affects respiratory health may be through the formation of O3. The NOx also secondarily form acidic nitrate particles.
NO2 is an oxidant gas of low solubility that penetrates to the small airways and alveoli of the lung. The toxicological evidence at exposures far greater than typically sustained in indoor and outdoor environments suggests that NO2 exposure can impair lung defenses against respiratory pathogens and cause airway inflammation, with associated effects on lung function and respiratory symptoms. In animal models, exposure to NO2 increases mortality after challenge with bacterial respiratory pathogens. Therefore, a wide range of health effects are of concern, including increased risk for respiratory infections, respiratory symptoms, reduced lung function, exacerbation of chronic respiratory diseases as well as acute cardiovascular responses including myocardial infarction. These studies and other evidence on indoor exposures are considered separately in the section on indoor air pollution. Several more recent studies link NO2 to indicators of morbidity, but the findings are inconsistent and are unlikely to reflect NO2 acting by itself. Interpretation of findings related to NO2 is complicated by its role in the formation of O3 and the importance of vehicular emissions as a source in most locations, making NO2 concentration a surrogate for the mixture of traffic-related pollutants.
A number of clinical studies have been performed to investigate the acute effects of NO2 by itself on persons with asthma.36 These studies were performed to assess the need for a short-term standard for outdoor NO2 concentration, as the present NAAQS provide only an annual standard for NO2. NO2 could plausibly affect airway responsiveness by causing airway inflammation. The findings of the clinical studies have been inconsistent, and the discrepancies between the “positive” and “negative” studies have not been readily explained. The EPA published its most recent assessment of the evidence in the form of an Integrated Science Assessment in 2008.37 That review concluded that NO2 is associated with increased airway responsiveness, citing the findings of epidemiological studies. As a result, NO2 exposure is thought to increase respiratory symptoms on a short-term basis and to contribute to respiratory morbidity. Persons with asthma or COPD may represent groups with increased susceptibility to short-term outdoor exposure to NO2.
Particles
Particles in outdoor air have numerous natural and artificial sources, including the same combustion processes that produce SO2 and NO2. Particles are suspended in air by the action of wind on crustal material and road dust. The artificial sources are diverse and include power plants, industry, and motor vehicles, including diesel-powered vehicles that emit particles in the inhalable size range. Particles, of course, are present in indoor and outdoor air. Consequently, personal exposures to particles reflect both indoor and outdoor microenvironments. In addition, outdoor particles, particularly those of smaller size, penetrate indoors.
The artificial particles are primary (i.e., emitted directly by combustion or other processes), or secondary (i.e., formed through chemical and physical transformation of gaseous pollutants, such as NO2 and SO2). Because of the diversity of sources, particles have a rich mixture/composition that may be quite variable, spatially and temporally. The toxicity of particles in a particular place thus reflects the source mix, which includes both local sources and those contributing to the regional background pollution (i.e., pollution generated elsewhere but transported to this and other locations via prevailing winds). In the Eastern United States, for example, much of the regional background of PM2.5 comes from transport of power plant emissions from the central portion of the country. Hypotheses proposed on the determinants of particle toxicity have focused on issues related to particle acidity, particle content of transition metals,38 organic compounds on particles (e.g., diesel particles), bioaerosols, and particle size (e.g., UFPs: particles less than 100 nm in aerodynamic diameter39; coarse particles [PM10–2.5]: particles between 2.5 and 10 μm in mass). Metals associated with particulate matter are capable of causing pulmonary inflammation and injury and the same chemical properties that allow metals to function as catalysts in reactions with molecular oxygen can generate oxygen-based free radicals and cause oxidative stress. UFPs have a high specific surface area and carry an increased burden of reactive oxygen species. They may be particularly important with regard to cardiovascular effects because of their potential for evading clearance mechanisms, and for entering the lung interstitium and vascular space. Coarse particles, by virtue of their size, are less likely to reach the alveoli. However, they have been associated with increased cardiovascular emergency department visits, and increased respiratory emergency department visits, with the most consistent evidence shown in children. Some, but not all, clinical studies and toxicological studies have also provided evidence for respiratory effects, including pulmonary inflammation and decreased pulmonary function.
Historically, particle concentrations in outdoor air have been measured with several different techniques and over recent decades these technologies have been refined and directed at biologically relevant size fractions. Until 1987, the EPA specified the measurement of total suspended particulates (TSPs), which included particles well above the inhalable size range. In 1987, the reference method for the NAAQS was changed to particulate matter less than 10 μm in aerodynamic diameter (PM10), and in 1997 24-hour and annual standards were added for PM2.5. The PM10 standard, challenged in court, was set aside in a Supreme Court decision, and in 2005, the EPA proposed a standard for coarse mixes in urban areas, PM10–2.5. This set of standards makes no provision with regard to the chemical composition of the particles. Equivalent mass concentrations of particles are hypothesized to have differing toxicity, depending on acidity, content of metals, or carcinogenic potency. The characteristics of particles that determine their toxicity are a focus of current research. The size distribution below the 10 and 2.5 μm cutoffs may also affect toxicity through its consequence for sites of deposition. In addition, the ultrafine component contributes little to the mass and the number count or surface area, but may turn out to be an important metric.
Nationally PM10 concentrations have decreased 31% since 1988, mainly in regions of the country that had higher concentrations such as the Northwest (39%), the Southwest (33%), and Southern California (35%). Since 1999, PM2.5 concentrations have decreased 10% nationally. PM2.5 has decreased the most in regions with the highest concentrations to start with, such as the Southeast (20%), Southern California (16%), and the industrial Midwest (9%). Except for the Northeast, most regions of the country have had at least modest declines from 1999 to 2003. Nationally these declines in PM10 and PM2.5 have continued through 2010 (Fig. 91-1).
A recent paper has suggested that these reductions in particulate air pollution during the 1980s and 1990s have led to measurable improvements in life expectancy in the United States.40 They estimated that for each 10 μg/m3 reduction in air pollution seen during this time in the United States (many areas in the country saw substantially larger reductions than this, and some regions smaller changes), the average gain in life expectancy was 0.61 years (7.3 months). This gain in life expectancy was independent of changes in socioeconomic status and other lifestyle factors. Further, they concluded that as much as 15% of the total life expectancy increase seen during this time in these areas, was attributable to the air pollution reductions.
There have been extensive epidemiological and toxicological investigations of the effects of particles on health since the air pollution disasters of mid-century.28,29 The toxicological studies have used a range of approaches, from exposing volunteers to generated particles or concentrated air particles, to animal exposures, and to diverse in vitro assays. This extensive body of evidence shows that particles are injurious and indicates mechanisms by which particles could cause adverse effects on the respiratory and cardiovascular systems.
The epidemiological studies have addressed the relationship between exposures to particles and short- and long-term variations in mortality, both from all causes and from cardiovascular and respiratory causes. The studies have also addressed the relationship between exposures to particles and diverse indicators of respiratory morbidity, including the frequency of respiratory symptoms and illnesses, level of lung function and rates of lung function growth and decline, and outpatient visits and inpatient admissions. More recently, studies have examined if and by what mechanisms particles may trigger acute cardiovascular and cerebrovascular events (e.g., myocardial infarction, ventricular arrhythmia, and stroke) in both epidemiology studies using outdoor particle concentrations (e.g., PM10 or PM2.5)41–43 and clinical studies using controlled particle exposures (e.g., concentrated UFP and concentrated PM2.5).44,45 These studies have identified numerous biomarkers of interacting mechanisms responding to short-term increases in ambient and controlled air pollution exposures including systemic and pulmonary inflammation, oxidative stress, coagulation, vascular dysfunction, and autonomic dysfunction to name a few. A more complete discussion of studies examining these and other mechanisms is provided in two recent American Heart Association statements on air pollution and cardiovascular disease.13,46 In the studies of particulate air pollution during the 1950s and 1960s, the measures of exposure were general. Some studies included only surrogate measures of exposure, such as location of the place of residence. In spite of such crude exposure measures, these studies found adverse effects of exposure to particulate air pollution and became the basis for establishing air quality standards for particles. The standards were generally considered to be sufficiently protective of public health. Studies linked particulate air pollution to a number of adverse health effects including total, cardiovascular, and respiratory mortality, exacerbation of asthma, hospital admissions, impaired lung function, and upper and lower respiratory symptoms.47
As levels of air pollution were reduced in the United States and other Western countries, excess mortality at times of higher concentrations was not readily evident and, since the 1970s, the focus of research and of public health concern has generally shifted to morbidity. However, studies of air pollution and daily variations in mortality, facilitated by new techniques for longitudinal data analysis, have shown statistically significant, positive associations between measures of particle concentration and daily mortality counts for cities in the United States and elsewhere. Several analyses have combined data for multiple cities in the United States, Europe, and Asia including studies in China.48–57 These analyses pool the information from many locations to estimate precisely the effect of particulate matter and also to examine the variations in risk among the contributing locations. These analyses show a statistically significant effect of airborne particles on mortality, independent of the possible contributions of other pollutants or weather. In interpreting these findings, the extent to which life is lost from these short-term effects is critical. Several prospective studies indicate that life shortening from air pollution may be substantial.
Many complementary studies of morbidity have also been reported. These studies have been directed at clinical indicators, such as hospitalization or the triggering of arrhythmias, myocardial infarction, or sudden death. They have also examined biomarkers and electrocardiographic parameters. A review in 2004,46 and an update in 2010,13 both by the American Heart Association, detailed a substantial body of evidence linking particulate air pollution to adverse cardiovascular effects. In addition, in some studies, airborne particles have been shown to adversely affect persons with asthma and COPD. These findings suggest that the present NAAQS for particulate matter may not protect against adverse health effects with the “adequate margin of safety” mandated by the Clean Air Act. They also call into question other national and international standards and have led to tightening of the standards, most recently in 2012 when the PM2.5 annual concentration standard was reduced to 12 μg/m3.
Guidance to patients related to ambient particulate air pollution calls for tracking when pollution levels are high, either by weather forecasts, EPA alerts, or visible haze and reduced visibility. At such times, it is reasonable to advise patients to reduce outdoor activity, particularly with exercise, and avoid locations where pollution levels may be particularly high, for example, adjacent to busy roadways. Such guidance is particularly appropriate for people susceptible to health effects of air pollution (e.g., people with asthma, COPD, coronary artery disease, and/or hypertension). Although mechanisms of toxicity are not completely understood, avoiding air pollution exposure can only help reduce air pollution-mediated cardiorespiratory morbidity and mortality.
Carbon Monoxide
CO is an invisible gas formed by incomplete combustion of fossil fuels and other organic materials. The most prominent outdoor source is vehicle exhaust; consequently, outdoor concentrations are highly variable in place and time, changing with vehicle density and traffic patterns. Urban locations with high traffic density tend to have the highest concentrations. CO also has indoor sources, such as cooking stoves and tobacco smoke. Exposures to CO can be conveniently assessed by using the level of carboxyhemoglobin (COHb) as a biomarker of exposure or by measuring the concentration of CO in an end-tidal breath sample, following a breath hold.
With the passage of the Clean Air Act, significant reductions in outdoor CO have occurred, reflecting emissions control particularly on vehicles. Between 1980 and 2010, there has been an 82% decrease in ambient CO levels. From 2000 to 2010, CO decreased 54%.58 In regard to outdoor air, acute effects of CO on susceptible persons have been of particular concern, and the current US standard is intended to protect susceptible persons with coronary artery disease. Inhaled CO binds to hemoglobin with high affinity (more than 200 times greater than for oxygen) to form COHb. The COHb complex is very stable; depending on ambient levels of CO, level of activity, and lung function, the half-life of CO in the body ranges from about 2.5 to 4 hours. The rate of accumulation of ambient CO in the body above endogenous levels is affected by ambient CO concentrations, alveolar ventilation, lung diffusivity, total hemoglobin mass, and COHb level.59 People with impaired gas exchange (e.g., persons with COPD) have compromised ability to excrete CO.
The binding of CO to hemoglobin reduces oxygen transport by red blood cells to tissues. The binding also displaces oxygen and causes an allosteric change in the hemoglobin molecule, which increases the affinity of heme groups for oxygen. Persons with cardiovascular disease are considered to be at greatest risk from CO exposure. Standard exercise tests on subjects with ischemic heart disease have demonstrated a decreased time interval to the onset of angina at COHb levels ranging from 2% to 6%.60 The 1-hour 35-ppm and 8-hour 9-ppm federal standards for outdoor air (Table 91-1) were selected to prevent COHb levels from rising above 1.5%, thereby protecting persons with ischemic heart disease from aggravation of myocardial ischemia with onset of angina and attendant loss of exercise capacity. Recent evidence indicates that controlled CO exposure during exercise of patients with stable coronary artery disease can induce subjective and objective evidence of myocardial ischemia earlier in the exposure than during exercise without CO. This effect can be induced at COHb levels as low as 2% to 4%. These studies are relevant to the urban environment, where people may be exposed to sufficient CO to reach blood COHb levels in this range. Furthermore, moderate exercise results in even greater CO uptake. In addition, at a COHb level of 6%, patients with coronary artery disease have an increased frequency of arrhythmias.61 Fetuses, as well as persons with COPD, may also be harmed by CO, and normal persons may have reduced oxygen uptake during exercise at low levels of CO exposure.61
The recent studies from the 2008 Beijing Olympics (discussed below) demonstrated a potential role of CO and perhaps other pollutants in inducing adverse changes in cardiorespiratory biomarkers in a panel of healthy young people. CO levels decreased 48% (together with similar-sized reductions in PM2.5, SO2, NO2, and other pollutants) from before to during the Olympic Games. These pollutant changes were associated with significant reductions in coagulation biomarkers suggesting a role in thrombosis endothelial dysfunction mechanisms43 as well as in exhaled breath biomarkers suggesting pollution-induced pulmonary inflammation along with signs of respiratory and systemic oxidative stress.62 A more recent study63 from Hong Kong found that lower CO levels were associated with a reduced risk of hospital admissions for respiratory tract infections. It is unclear whether pollutant-related CO exposure causes noncardiac toxicity independently or whether it plays a role in health effects elicited by the air pollution mixture.
Ozone
Photochemical pollution, or “smog,” is a complex oxidant mixture produced by the action of sunlight on hydrocarbons and NOx in vehicle exhaust.28,29 O3, a secondary pollutant, is invariably present in photochemical pollution, and its concentration serves as an index of the level of this mixture. The problem of tropospheric O3 pollution, that is, ground level, is distinct from the problem of depletion of the stratospheric ozone layer. Photochemical pollution was first recognized over 50 years ago in southern California, where the combination of sunlight and heavy vehicle travel promotes its formation. O3 has now become a problem in many other locations, including Western cities with similar sprawling growth and heavy vehicle traffic and the Eastern United States during the summer. O3 is also produced naturally, but the exposure of concern for health almost exclusively reflects the O3 created by human activities.
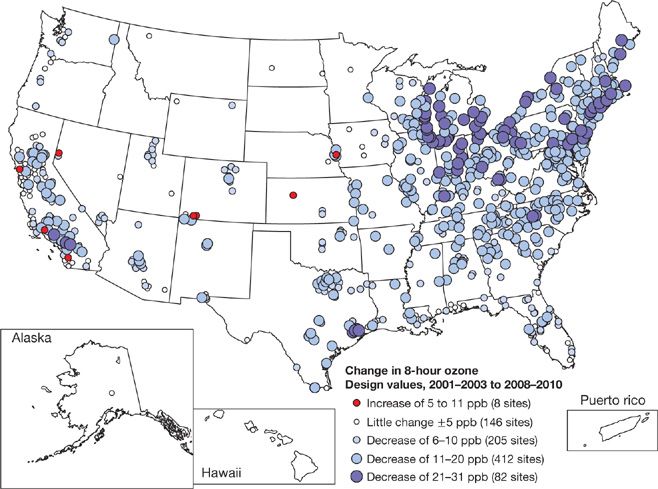
Since 1990, NOx emissions have decreased approximately 25% and VOCs emissions have dropped by about 35%. National ozone levels in 2004 were 11% lower than in 1990 and 21% lower than in 1980 for the 8-hour standard (3-year average of the annual fourth highest daily maximum 8-hour average concentration is less than 0.08 ppm). Between 1990 and 2004 the most significant improvements in air quality were in the Northeast (17% decrease) and Southwest (16% decrease) regions of the country.64 This decline has continued through 2010 for most of the country, with the larger declines in the Northeast (Fig. 91-3).
Figure 91-3 Individual monitor 8-hour daily maximum ozone design values, displayed as change from 2001–2003 to 2008–2010.
The toxicology of O3 has been extensively investigated. Low-level exposures cause damage to the small airways of experimental animals; the demonstration of subtle fibrosis in one animal model has raised concern about permanent structural alteration in exposed populations. Volunteers exposed to O3 at concentrations in the range of the present standard – which are often present during pollution episodes – experience transient reductions in lung function; normal subjects have a range of responsiveness that is broad but repeatable for individuals. Evidence of an inflammatory response and biochemical changes in BAL fluid has been detected 18 hours after an experimental exposure to O3 at levels that are commonly found. Taken together, the progressive decrements in pulmonary mechanics during exposure, coupled with the persistent biochemical changes many hours after cessation of exposure, indicate the potential for chronic effects from repeated inhalation. Surprisingly, in clinical studies, asthmatics have not been shown to have increased susceptibility to O3 compared with nonasthmatics. The evidence for short-term effects of O3 exposure on the lung function of normal volunteers has raised concern about possible long-term effects of living in Southern California and other locations with sustained photochemical pollution. Relevant epidemiological data suggest that O3 may have chronic effects, but these data are not definitive, and a long-term study of southern California children found that O3 specifically was not associated with reduced lung function growth, although other measures of the air pollution mixture were.25,65 The same study found evidence that O3 might contribute to the onset of asthma. Some time-series studies have linked short-term exposure to O3 to increased risk of mortality.66
Although there is some evidence that long-term O3 exposure is associated with increased respiratory and cardiovascular mortality, less is known about if and how O3 may impact cardiovascular morbidity. Epidemiology studies have provided inconsistent evidence, with one study reporting increased risk of paroxysmal atrial fibrillation associated with increased O3 concentrations67 while several studies of myocardial infarction find no such associations with increased O3 concentrations.68 There have been few clinical studies of cardiovascular health effects of O3 exposure or concentrations.
Recently, a clinical study using “low levels” of O3 (0.06 and 0.07 ppm for 6.6 hours with exercise) found possible lung function declines following O3 exposure.69 A second study with the same two levels of O3 exposure reported decreased lung function at 0.07 ppm, but not at 0.06 ppm.70 Based in part on these studies, the 8-hour O3 standard was reduced to 0.075 ppm. However, there are no studies of cardiovascular responses at these low levels.
Mixtures—Traffic and Diesel Pollution
Much of the health effects research that we discuss in this chapter is based on studies examining individual pollutants, one at a time. For example, in the clinical studies, investigators contrast two exposures (e.g., UFPs at a high concentration with no other pollutants vs. clean air with no UFP or other pollutants) so as to isolate health effects of UFP exposure. In epidemiology studies, researchers typically estimate the risk of a health event associated with an increase in the concentration of one pollutant, even though it is part of a complex mixture. However, none of these pollutants exist in the natural world in isolation. Each day, we are exposed to mixtures of pollutants in various places (at home while cooking food, in a car on the highway, at work in an office or manufacturing facility, etc.). Recently, researchers have begun to look at these air pollution mixtures, both in controlled exposure and epidemiology studies. A review of traffic pollution and related health effects has recently been published.7
For example, researchers have studied whether traffic pollution exposure (i.e., gasoline engine emissions, resuspended road dust, tire wear, brake wear) is associated with cardiovascular and respiratory morbidity. One approach for investigating such health effects of traffic pollution is a panel study of people who commute, with health measurements (e.g., blood sample, ECG recording) before, during, and after the time a subject is in the car. Simultaneously, the air pollution levels in the car can be measured, and in statistical analyses, one can determine if the in-vehicle air pollutant levels, which the subject presumably inhaled, were associated with adverse changes in markers measured in the blood and on the ECG. In these panel studies, increased in-vehicle pollution has been associated with adverse changes in numerous cardiovascular biomarkers of inflammation, vascular function, and autonomic function.71–74 In epidemiological studies, an increased risk of myocardial infarction or ventricular arrhythmias has been associated with being in a car in the previous few hours75 or even the previous 30 minutes,76 and with residential distance to major roadways77 (with people living closer to highways assume to have higher traffic pollution exposure than people living farther from highways). Individual pollutants thought to be markers of traffic pollution (e.g., NO2, CO, and black carbon) have repeatedly been associated with both cardiovascular and respiratory events, and also with adverse changes in these biomarkers of mechanisms thought to underlie the cardiorespiratory response to pollution. Clinically controlled exposure studies presumably provide the cleanest assessment of health effects of traffic pollution. Evidence clearly suggests that traffic pollution has adverse health effects. However, there is concern that stress and road noise, which may occur at the same time as traffic pollution exposure, may confound these associations.
The impact of diesel exhaust exposure remains another area of concern for adverse cardiopulmonary effects including lung cancer, asthma, and respiratory infections. Several research groups have conducted controlled exposure studies of diesel exhaust, with mixed results. Diesel exhaust is an irritant to which some people are highly sensitive. It has been classified as a human carcinogen by the International Agency for Research on Cancer (IARC), which has concluded that it causes lung cancer. The recently reported Advanced Collaborative Emissions Study (ACES) investigated the cancer and noncancer health effects of subchronic exposures to rats and mice for up to 12 months to diesel exhaust emissions from a heavy-duty diesel engine system compliant with 2007 EPA regulations.78 In brief, only mild exposure-related effects from detailed histology, pulmonary function, and blood biomarker studies were found. In contrast to the pollutant levels in the “traditional diesel,” the levels of PM, SO2, and VOCs in the “new” diesel engines were quite low. A future report on exposures up to 24 months should provide additional insight regarding toxicity of diesel exhaust.
In summary, research methods to study the health effects of these traffic pollutant mixtures, in animal, clinical, and population settings, are being refined. As such, our ability to understand if and how these mixtures impact our health will be greatly improved in the years to come. More work is needed to confirm these adverse effects of traffic, but clearly reducing time exposed to vehicle exhaust would be beneficial.
Lead
Exposure to lead may occur through many environmental media, including ambient air. At present, ingestion is the principal pathway of concern in the United States. Fortunately, in the United States the importance of ambient air as a source of exposure of the population to lead has diminished with the removal of lead from gasoline. Children are particularly vulnerable to lead exposure. Even levels previously considered safe have been associated with adverse neurological effects, and there has been a progressive tightening of recommendations of blood lead levels by the Centers for Disease Control and Prevention. More recent data have linked lead exposure with osteoporosis and possible arthritis.79 In adults, bone mineral density has shown an inverse association with blood lead levels.80
Toxic Air Pollutants
The toxic air pollutants are predominantly carcinogens, but they also include a variety of other toxins. Approximately 200 “hazardous pollutants” are listed as air toxins in the 1990 Clean Air Act amendments. Examples of the hazardous pollutants are asbestos, benzene, cadmium compounds, chlorine, formaldehyde, and nickel. Although the sources are diverse, emission releases tend to be localized, often at industrial sites, or from municipal incinerators or waste sites. The health consequences of these agents are diverse, and include cancer and noncancer effects. Formaldehyde, a ubiquitous pollutant in urban areas, causes cancer of the nasopharynx, exacerbates asthma, and is an irritant to the eyes and upper airway.
Only a small proportion of lung cancers can be attributed to air pollution, even though carcinogens are found widely in outdoor air. For example, polycyclic aromatic hydrocarbons (PAHs), in diesel exhaust, are widely dispersed and present in urban air throughout the world. The PAHs possess mutagenic and carcinogenic activity. But, to date, only limited epidemiological data on risks in humans are available. Analyses of occupational cohorts exposed to diesel exhaust for years are suggestive of a small excess risk of lung cancer.81 The IARC of the World Health Organization has classified diesel particles as a human carcinogen.82 Given the difficulties of measuring or estimating exposure, confounding by cigarette smoke and by other occupations, and the small excess numbers of lung cancers, it is difficult to quantify the risk of lung cancer associated with diesel exhaust exposure with a high degree of certainty. Nevertheless, as the percentage of light-duty vehicles powered by diesel fuel in the United States increases, there will be an increasing imperative to determine the carcinogenicity of the PAHs and diesel exhaust. There is also current concern that new types of fuels may introduce additional carcinogens into outdoor air. However, on the more positive side, the ACES discussed earlier has demonstrated the potential with engineering controls to dramatically reduce emissions even from the heavy-duty diesel engines and their potential adverse health effects.78
INDOOR AIR POLLUTANTS AND HEALTH EFFECTS
Indoor environments are contaminated by numerous air pollutants, including outdoor air pollutants that have penetrated indoors and indoor air pollutants generated by the numerous indoor sources. This section reviews exposures and health effects of the principal indoor pollutants. The organic compounds and biological agents include myriad individual agents that may adversely affect health. As for outdoor air pollution, clinicians should consider that exposures to indoor air pollutants typically occur as exposures to mixtures, rather than as single agents.
 OVERVIEW: SOURCES AND CLASSIFICATION OF INDOOR AIR POLLUTION
OVERVIEW: SOURCES AND CLASSIFICATION OF INDOOR AIR POLLUTION
Indoor air pollution has myriad sources, including the materials from which the space is constructed, its furnishings, processes operating within the environment, biological agents, and even the occupants. Outdoor air pollutants can also penetrate indoors, as can soil gas, which may contain radon and termiticides. The broad source headings are combustion, evaporation, abrasion, biological, and radon (Table 91-4). The principal combustion sources are gas cooking stoves, burning cigarettes, fireplaces and wood stoves, and unvented space heaters. Evaporation of VOCs from materials and products leads to ubiquitous contamination by these agents. Abrasion of friable asbestos is a principal source of this indoor contaminant. The biological agents are heterogeneous, extending from infectious organisms to pets and the occupants themselves. Radon comes primarily from soil gas.