Modulators of normal bodily functions such as the duration and quality of sleep might transiently influence cardiovascular risk. The transition to daylight savings time (DST) has been associated with a short-term increased incidence ratio (IR) of acute myocardial infarction (AMI). The present retrospective study examined the IR of AMIs that presented to our hospitals the week after DST and after the autumn switch to standard time, October 2006 to April 2012, with specific reference to the AMI type. Our study population (n = 935 patients; 59% men, 41% women) was obtained from the electronic medical records of the Royal Oak and Troy campuses of the Beaumont Hospitals in Michigan. Overall, the frequency of AMI was similar in the spring and autumn, 463 (49.5%) and 472 (50.5%), respectively. The IR for the first week after the spring shift was 1.17 (95% confidence interval 1.00 to 1.36). After the transition from DST in the autumn, the IR for the same period was lower, but not significantly different, 0.99 (95% confidence interval 0.85 to 1.16). Nevertheless, the greatest increase in AMI occurred on the first day (Sunday) after the spring shift to DST (1.71, 95% confidence interval 1.09 to 2.02; p <0.05). Also, a significantly greater incidence was found of non–ST-segment myocardial infarction after the transition to DST in the study group compared with that in the control group (p = 0.022). In conclusion, these data suggest that shifts to and from DST might transiently affect the incidence and type of acute cardiac events, albeit modestly.
Several studies have noted circadian and seasonal patterns for acute myocardial infarction (AMI). In general, AMIs are more likely to occur in the early to late morning hours and during the winter months. In 2008, Janszky and Ljung reported a 5% to 10% increased incidence of AMIs the week after daylight savings time (DST) in the spring, and, in the autumn, a pronounced decrease in AMIs on the Monday after the switch to standard time. To identify the higher risk subgroups who might benefit from avoiding even modest sleep deprivation, these investigators subsequently calculated the incidence ratios (IRs) in strata defined by varied patient characteristics. Both reports, derived from the National Swedish Myocardial Infarction Register and the Register of Information and Knowledge about Swedish Heart Intensive Care Admission, respectively, acknowledged numerous study limitations and potential confounding variables (e.g., the delay in coronary care unit admissions). Moreover, it remains unclear to what extent these findings are generalizable to other countries, with different latitudes, environmental/seasonal conditions, lifestyle practices, and healthcare systems. The present study retrospectively examined the number of AMIs that presented to our hospitals the week after the shifts to and from DST and classified them according to myocardial infarction type (ST-segment elevation myocardial infarction [STEMI] versus non–ST-segment elevation myocardial infarction [NSTEMI]).
Methods
The present study was a retrospective electronic chart review of all patients presenting to the emergency centers at Beaumont Hospitals in Royal Oak and Troy, Michigan, with the primary diagnosis of AMI the week after the spring transition to DST and the week after the shift back to standard time in the autumn from October 2006 to April 2012. The timing of AMI was the time the patient presented to the hospitals’ emergency center. Patients admitted with comparable diagnoses on the corresponding weekdays 2 weeks before and 2 weeks after the shifts to and from DST were included in the analysis and served as controls.
The electronic charts were reviewed to obtain the demographic data, medical history, tobacco use, prescribed medications, and whether the patient underwent cardiac catheterization. The diagnosis of hypertension, hyperlipidemia, and coronary artery disease were determined by documentation in the electronic medical chart. Medication use was obtained from either the dictated history and physical examination findings, consultation, or previous medications listed in the emergency center note. When admission medications were not available, those listed in a summary dictation within the previous year were used. Smoking status was characterized as never, current, or former smoker. If a patient underwent cardiac catheterization, the culprit vessel was recorded, or, if no intervention was performed, the underlying clinical rationale was documented. The reasons for not undergoing cardiac catheterization were also recorded and determined by the dictated reports and included age, comorbidities, patient preference or refusal, and medical treatment chosen by the attending cardiologist. The patients were listed as having a family history of coronary artery disease if noted as such in the medical record or if first-degree family members (age <70 years) had coronary disease.
An Internet search using Google was performed to identify the dates of the shifts to and from DST in Michigan from 2006 to 2012. An online calendar was then used to identify each of the 7 days after the spring or autumn transition and the corresponding weekdays 2 weeks before and 2 weeks after the time shifts. The patients admitted with the primary diagnosis of AMI who were aged >18 years were included in the present study. The patients were excluded if they were minors or pregnant or if their dictated reports clearly stated that the patient had not experienced an AMI and that the cardiac enzyme elevation was from another cause.
The overall IR of AMI was calculated by comparing the incidence of AMI during each of the first 7 days after the spring or autumn transition (observed incidence) and the mean of the incidence on the corresponding weekdays 2 weeks before and 2 weeks after the day of interest (expected incidence). The expected incidence on any given day was calculated by dividing the incidence on that day by the mean incidence on the corresponding weekday 2 weeks earlier and 2 weeks later. The number of AMIs on the transition Sunday was adjusted for the difference in day length compared with the control Sundays (i.e., 23 vs 24 hours in the spring and 25 vs 24 hours in the autumn). SAS for Windows, version 9.2 (SAS Institute, Cary, North Carolina), was used for all analyses. Two patients experienced 2 AMIs within 30 days. We only included their first AMI. Also, 2 AMIs occurred on Easter Sunday and were considered potential confounders and excluded. The “cinv” function in SAS, a method based on the relation between the Poisson distribution and the chi-square distribution, was used to create 95% confidence intervals (CIs).
Results
A total of 935 patients (551 men [59%], 384 women [41%]; mean ± SD age 70.0 ± 14.8 years, range 29 to 106) were included in the study population. Of the 328 patients in the study group, 206 were men and 122 were women; in the control group, 347 were men and 260 were women. No significant differences were found between the 2 groups for demographics, gender, risk factors, tobacco use, or prescribed medications ( Table 1 ). Overall, the frequency of AMI was similar in the spring and autumn, 463 (49.5%) and 472 (50.5%), respectively. The IR for the first week after the spring shift was 1.17 (95% CI 1.00 to 1.36). In contrast, after the transition from DST in the autumn, the IR for the same period was slightly lower, but not significantly different (0.99, 95% CI 0.85 to 1.16). When analyzing spring and autumn combined, the IR was 1.08 (95% CI 0.96 to 1.12).
| Variable | Control Group (n = 607) | Study Group (n = 328) | p Value |
|---|---|---|---|
| Year | |||
| 2006 | 70 (11.5%) | 27 (8.2%) | 0.29 |
| 2007 | 94 (15.5%) | 57 (17.4%) | |
| 2008 | 91 (15.0%) | 48 (14.6%) | |
| 2009 | 109 (18.0%) | 48 (14.6%) | |
| 2010 | 80 (13.2%) | 58 (17.7%) | |
| 2011 | 101 (16.6%) | 59 (18.0%) | |
| 2012 | 62 (10.2%) | 31 (9.5%) | |
| Age (yrs) | 71 ± 14 | 69 ± 15 | 0.12 |
| Women | 260 (43%) | 122 (37%) | 0.09 |
| Body mass index (kg/m 2 ) | 29.4 ± 7.1 | 28.7 ± 6.1 | 0.39 |
| Diabetes mellitus | 193 (32%) | 97 (30%) | 0.48 |
| Hypertension | 436 (72%) | 238 (73%) | 0.79 |
| Hypercholesterolemia | 317 (53%) | 165 (50%) | 0.54 |
| Previous coronary artery disease | 274 (45%) | 162 (50%) | 0.21 |
| Previous coronary bypass | 82 (13.5%) | 59 (18%) | 0.07 |
| Previous percutaneous coronary intervention | 203 (34%) | 120 (37%) | 0.32 |
| Previous peripheral vascular disease | 107 (18%) | 49 (15%) | 0.29 |
| Previous cardiovascular accident | 79 (13%) | 40 (12%) | 0.70 |
| Previous arrhythmias | 96 (16%) | 57 (17%) | 0.55 |
| Sleep apnea | 22 (3.6%) | 16 (4.9) | 0.35 |
| Smoking status | |||
| Previous | 156 (26%) | 86 (26%) | 0.89 |
| Current | 88 (15%) | 56 (17%) | 0.30 |
| Family history of coronary artery disease | 100 (17%) | 71 (22%) | 0.06 |
| Medications | |||
| Aspirin | 300/580 (52%) | 161/316 (51%) | 0.82 |
| Antiplatelet therapy | 112/579 (19%) | 69/315 (22%) | 0.36 |
| β Blockers | 272/579 (47%) | 155/316 (49%) | 0.55 |
| Calcium blockers | 136/581 (23%) | 67/314 (21%) | 0.48 |
| Angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers | 246/579 (42%) | 127/314 (40%) | 0.55 |
| Statins | 258/580 (44%) | 130/314 (41%) | 0.37 |
| Ezetimibe | 32/579 (5.5%) | 18/314 (5.7%) | 0.90 |
| Diuretics | 188 (32%) | 94 (30%) | 0.44 |
| Nitrates | 77/579 (13%) | 54/315 (17%) | 0.12 |
| Insulin | 75/580 (13%) | 39/314 (12%) | 0.83 |
| Oral antiglycemic agents | 88 (15%) | 48 (15%) | 0.97 |
| Warfarin/dabigatran | 33/579 (5.7%) | 18/314 (5.7%) | 0.98 |
| Cilostazol | 3/579 (0.5%) | 5/314 (1.6%) | 0.14 |
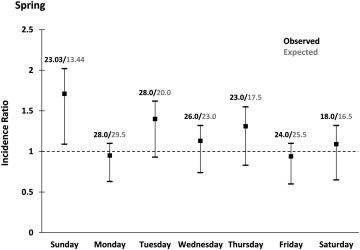
The spring cohort included 463 patients, 292 (mean ± SD age 70.0 ± 15.0 years) in the control group and 171 (mean ± SD age 69.0 ± 16.0 years) in the study group. In general, no differences were found in risk factors, previous coronary revascularization procedures, or medications between the groups ( Table 1 ). The IR for the first week after the spring shift to DST (1.17, 95% CI 1.00 to 1.26) was not significantly different than that for the same period after the transition from DST in the autumn (0.99, 95% CI 0.85 to 1.16). However, the largest increase in AMI occurred on the first day (Sunday) after the spring shift (1.71, 95% CI 1.09 to 2.02; p <0.05). During the subsequent 6 days, the IRs were unremarkable ( Figure 1 ). When evaluating patients with AMI on Sunday, a greater use of calcium channel blockers was found in the study group compared with that in the control group (p = 0.017). A significantly greater incidence of NSTEMI also occurred after the transition to DST in the spring study group (p = 0.022) compared with the control group ( Table 2 and Figure 2 ).