Stent thrombosis (ST) remains a catastrophic problem in patients undergoing percutaneous coronary intervention (PCI). However, a paucity of data exist regarding the incidence, implications, and predictors of ST in patients with acute myocardial infarction (AMI). We consecutively enrolled patients with AMI in the CardiOvascular Risk and idEntificAtion of potential high-risk population in AMI registry who underwent PCI from January 2004 to December 2009 and analyzed definite or probable ST according to Academic Research Consortium definitions. The median follow-up duration was 41.9 months. Definite or probable ST occurred in 136 patients (3.7%), including 44 with early ST (1.0%), 38 with late ST (0.9%), and 54 with very late ST (2.0%). The annual incidence of very late ST ranged from 0.5% to 0.6%. The all-cause mortality rate after ST was 29%, which was higher than that for patients without ST (17%; p <0.001). The independent predictors of ST were no-reflow phenomenon (hazard ratio [HR] 1.96, 95% confidence interval [CI] 1.28 to 3.03), decreased left ventricular ejection fraction (HR 1.70, 95% CI 1.21 to 2.40), anemia (HR 1.54, 95% CI 1.09 to 2.18), and a mean stent diameter <3.0 mm (HR 1.53, 95% CI 1.04 to 2.27). ST is not uncommon in patients with AMI and continues to occur beyond 1 year after PCI, irrespective of the stent type or clinical presentation. Patients with ST are associated with higher mortality than patients without ST. No reflow, decreased left ventricular ejection fraction, anemia, and a mean stent diameter <3.0 mm are independent predictors of ST.
Stent thrombosis (ST) is a rare but fatal complication of percutaneous coronary intervention (PCI). In the past decade, although PCI has been associated with improved clinical outcomes, concern about the risk for ST has been raised, and ST has remained a considerable problem. The incidence of ST differs according to variable clinical settings and is reported to be approximately 1% to 2% according to many randomized trials and registries handling subjects with mostly stable coronary artery disease (CAD). However, a paucity of data are available regarding ST after PCI in acute myocardial infarction (AMI), and there is a lack of clinical research to evaluate the long-term mortality of ST in patients with AMI. Because of the small number of trials conducted and the low incidence of ST, the actual incidence and predictors of ST in patients with AMI undergoing stent implantation remain unclear. Furthermore, although very late stent thrombosis (VLST), which is defined as ST occurring >1 year after index PCI, remains worrisome to patients, few studies have focused on the incidence or specific predictors of VLST in patients with AMI. Therefore, we investigated the incidence, clinical implications, and predictors for the development of ST, especially VLST, in a large real-world AMI registry.
Methods
The CardiOvascular Risk and idEntificAtion of potential high-risk population in AMI registry was established to evaluate the real-world outcomes in all patients with AMI. Consecutive patients who were admitted to 1 of 9 nationwide hospitals, which were high-volume PCI centers, and diagnosed with AMI were prospectively enrolled from January 2004 to December 2009. All the enrolled patients were aged >20 years and were treated with PCI using either drug-eluting stents (DES) or bare-metal stents (BMS). Patients managed with a conservative strategy were excluded. In the present study, patients who had received heterogeneous stents, including both BMS and DES within the culprit vessel, were excluded.
AMI was diagnosed by detecting the rise and/or fall of cardiac biomarkers, with at least 1 value above the ninety-ninth percentile, along with at least 1 of the following indications: symptoms of ischemia, new or presumed-new significant ST-T changes or new left bundle branch block, development of pathologic Q waves on electrocardiography, imaging evidence of new viable myocardium loss or new regional wall motion abnormality, or intracoronary thrombus identified by angiography. The clinical presentation was divided into 2 groups: ST-segment elevation myocardial infarction (STEMI) and non–ST-segment elevation myocardial infarction (NSTEMI). New or presumed-new ST-segment elevation, new left bundle branch block, or isolated inferobasal myocardial infarction (MI) before any procedures indicated STEMI, and the other cases were regarded as NSTEMI. The stent type was classified as DES or BMS according to the type of stent implanted in the culprit vessel.
Clinical and outcome data were collected by independent research personnel, and angiographic and procedural data were assessed and entered by an independent interventional cardiologist. Each patient was followed up during an office visit or telephone conversation. All the outcomes of interest were confirmed by a source document and were centrally adjudicated by a local events committee of the Cardiovascular Center of Seoul St Mary’s Hospital, Seoul, Republic of Korea; the committee members were unaware of the statuses of patients. To validate the complete follow-up data, information on censored survival data and causes of death was obtained from the Korea National Statistics Office using unique patient identification numbers. The study was conducted in compliance with the Declaration of Helsinki regarding investigations in humans, and written informed consent was obtained from each patient before enrollment. The study protocol was approved by the Institutional Review Board at each participating hospital. This registry has been registered on ClinicalTrials.gov (study ID: NCT02385682 ).
Aspirin- or clopidogrel-naive patients received loading doses of 250 to 500 mg of aspirin and 600 mg of clopidogrel. Heparin was used before and at the time of PCI, and a glycoprotein IIb/IIIa inhibitor was administered at the discretion of a physician. Newer antiplatelet agents, such as prasugrel, ticagrelor, and bivalirudin, were not available during the period in which this study was conducted. Stent implantation was performed according to a standard technique, and the stent type was chosen at the discretion of the operator. Most patients received dual-antiplatelet therapy; other medications, including β-blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and HMG-CoA (3-hydroxy-3-methylglutaryl-coenzyme A) reductase inhibitors, were administered depending on patient condition.
An independent angiographic core laboratory assessed all initial and follow-up angiograms; the assessors were blinded to baseline information and clinical events during follow-up. The coronary flow grade was determined according to the thrombolysis in myocardial infarction (TIMI) classification. The no-reflow phenomenon was defined as angiographic documentation of a TIMI flow grade ≤2 at least 10 minutes after PCI, with no evidence of flow-limiting residual stenosis (<50%), dissection, spasm, or apparent thrombus.
ST was defined as definite or probable ST according to the Academic Research Consortium definitions. Definite ST was diagnosed by angiographic confirmation of ST when acute coronary syndrome (ACS) with a nonocclusive or an occlusive thrombus within the stent was diagnosed. Probable ST was defined as any unexplained sudden death within 30 days or any MI within the territory of the implanted stent without angiographic confirmation, irrespective of the time after the index procedure. ST was classified according to the timing of its occurrence. Early ST was defined as ST occurring from 0 to 30 days after the index PCI, whereas late ST occurred from 31 to 365 days after index PCI, and VLST occurred more than 1 year after index PCI.
All analyses were 2 tailed, and clinical significance was defined as a p value <0.05. Statistical analyses were performed using SAS software version 9.8 (SAS Institute, Cary, North Carolina). To detect differences between patients with ST and without ST, baseline characteristics were analyzed by the Student t test or the Mann–Whitney U test for continuous variables after testing for normality and by a chi-square test or Fisher’s exact test for categorical variables, as appropriate. The incidence of ST was assessed by Kaplan–Meier estimates at 30 days, 1 year, and annually up to 5 years after the index PCI. Survival curves were obtained by Kaplan–Meier analysis and were compared using the log-rank test. Mortality rates in the ST group were analyzed from the occurrence of ST, and those in the no-ST group were analyzed from the index PCI. Multivariate analyses to evaluate the predictors of ST were performed with Cox proportional hazards regression models. Because of their clinical relevance, diabetes, stent type, clinical presentation, and all clinical and angiographic variables with p values <0.1 by univariate analyses were entered into the models. Landmark analyses were performed to analyze the predictors of early ST, late ST, and VLST. Patients who survived without ST for 30 days and 1 year were included in the landmark analyses for late ST and VLST, respectively.
The proportion of missing values was 2% (112 of 4,720 patients) for body mass index, 4% (184 of 4,720) for hyperlipidemia, 0.1% (6 of 4,720) for serum creatinine, 0.1% (4 of 4,720) for Killip class, 3% (153 of 4,720) for left ventricular ejection fraction (LVEF), 1% (48 of 4,720) for hemoglobin, 0.6% (29 of 4,720) for platelet count, 11% (524 of 4,720) for high-sensitivity C-reactive protein, 0.04% (2 of 4,720) for discharge medication, 0.3% (14 of 4,720) for initial TIMI flow, 0.04% (2 of 4,720) for mean stent diameter, and 1% (57 of 4,720) for total stent length. No data were missing for variables other than those mentioned previously. Available information was included in the statistical analyses without the distortion resulting from using imputed values for missing data.
Results
From January 2004 to December 2009, a total of 4,748 patients with AMI underwent PCI. Twenty-eight patients who had received heterogeneous stents were excluded from the analyses. With a median follow-up of 42 months (interquartile range [IQR] 27 to 59 months), 136 patients were diagnosed with ST (Kaplan–Meier estimated rate, 3.7%); 110 of these cases of ST were definite, and 26 were probable. All definite ST cases were confirmed by coronary angiography. The subjects’ baseline clinical and angiographic characteristics are summarized in Tables 1 and 2 . Patients with ST showed a lower LVEF ( Table 1 ) and a higher frequency of no reflow ( Table 2 ). Baseline characteristics according to the stent type or the clinical presentation are provided in Supplementary Tables 1 and 2 .
| Variables | Stent thrombosis | p Value | |
|---|---|---|---|
| Yes (n= 136) | No (n= 4584) | ||
| Age (years) | 62.0±13.4 | 62.6±12.5 | 0.59 |
| Men | 93 (68%) | 3292 (72%) | 0.38 |
| Body mass index (kg/m 2 ) | 23.9±3.3 | 24.2±3.2 | 0.24 |
| Hypertension | 64 (47%) | 2283 (50%) | 0.47 |
| Diabetes mellitus | 42 (31%) | 1439 (31%) | 0.90 |
| Hyperlipidemia ∗ | 37/133 (28%) | 1295/4403 (29%) | 0.69 |
| Current smoker | 62 (46%) | 1958 (43%) | 0.50 |
| Renal insufficiency † | 40 (29%) | 1082/4578 (24%) | 0.12 |
| Previous myocardial infarction | 9 (7%) | 164 (4%) | 0.10 |
| Previous percutaneous coronary intervention | 6 (4%) | 181 (4%) | 0.79 |
| Killip class III∼IV | 20 (15%) | 519/4580 (11%) | 0.22 |
| Left ventricle ejection fraction | 50.6±11.5 | 53.8±11.7 | 0.001 |
| Hemoglobin (g/dL) | 13.0±2.2 | 13.3±2.4 | 0.13 |
| Platelet (10 3 cells/mm 3 ) | 246±125 | 225±65 | 0.21 |
| Hs-C-reactive protein (mg/L) | 28.4±44.5 | 23.2±40.9 | 0.17 |
| Statin at discharge | 109/124 (88%) | 3973/4480 (87%) | 0.79 |
| Clinical presentation | 0.19 | ||
| STEMI | 91 (67%) | 2812 (61%) | |
| NSTEMI | 45 (33%) | 1772 (39%) | |
∗ Total cholesterol ≥200mg/dL.
† Estimated glomerular filtration rate using modification of diet in renal disease formula <60 mL/min/1.73m 2 .
| Variables | Stent thrombosis | p Value | |
|---|---|---|---|
| Yes (n= 136) | No (n= 4584) | ||
| Number of coronary arteries involved | 0.82 | ||
| 1 | 64 (47%) | 2198 (48%) | |
| 2 | 40 (29%) | 1343 (29%) | |
| 3 | 28 (21%) | 905 (20%) | |
| Left main | 4 (3%) | 138 (3%) | |
| Culprit coronary artery | 0.97 | ||
| Left anterior descending | 67 (49%) | 2198 (48%) | |
| Left circumflex | 24 (18%) | 740 (16%) | |
| Right coronary | 43 (32%) | 1540 (34%) | |
| Left main | 2 (1%) | 103 (2%) | |
| Baseline thrombolysis in myocardial infarction flow 0∼1 | 67/135 (50%) | 2252/4571 (49%) | 0.93 |
| Stent number of culprit vessel | 1.1±0.4 | 1.1±0.4 | 0.69 |
| Stent diameter of culprit vessel | 3.2±0.5 | 3.2±0.4 | 0.13 |
| Stent length of culprit vessel | 26.8±13.1 | 27.3±12.3 | 0.60 |
| Use of intravascular ultrasound | 41 (30%) | 1261 (28%) | 0.50 |
| Use of glycoprotein IIb/IIIa inhibitor | 31 (23%) | 855 (19%) | 0.22 |
| No reflow phenomenon | 27 (20%) | 623 (14%) | 0.04 |
| Type of stent | 0.24 | ||
| Bare-metal stent | 17 (12%) | 434 (9%) | |
| Drug-eluting stent | 119 (88%) | 4150 (91%) | |
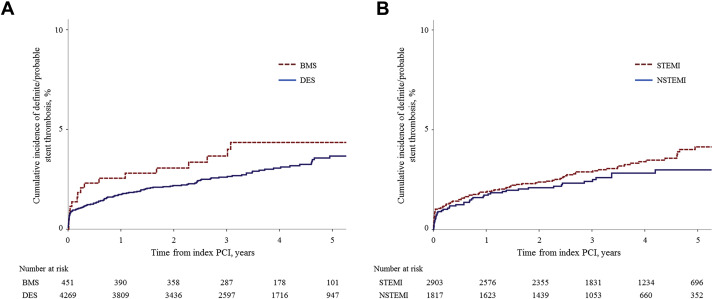
The incidence of early ST was 1.0% (29 definite and 15 probable ST), whereas the incidence of late ST was 0.9% (37 definite and 1 probable ST), and the incidence of VLST was 2.0% (44 definite and 10 probable ST). As estimated by landmark analysis using Kaplan–Meier estimates, the annual incidence of VLST ranged from 0.5% to 0.6%/year ( Table 3 ). Of the 4,720 study patients, 451 patients received only BMS, whereas 4,269 received only DES. No significant differences were found in follow-up duration according to the stent type: BMS median 41.6 months (IQR 26.9 to 57.1 months) versus DES median 42.1 months (IQR 27.4 to 59.0 months; p = 0.65). Similar incidences of ST were observed for total ST, early ST, late ST, and VLST ( Table 3 and Figure 1 ). As estimated by landmark analysis, the annual incidence of VLST ranged from 0.0% to 0.7% per year in the BMS group versus 0.4% to 0.6% per year in the DES group. In addition, no significant differences were found between the 2 clinical presentations for the incidences of total ST, early ST, late ST, and VLST ( Table 3 and Figure 1 ).
| All patients (n=4720) | BMS (n=451) | DES (n=4269) | p Value (BMS vs. DES) | STEMI (n=2903) | NSTEMI (n=1817) | p Value (STEMI vs. NSTEMI) | |
|---|---|---|---|---|---|---|---|
| All stent thrombosis | 136 (3.7%) | 17 (4.4%) | 119 (3.7%) | 0.23 | 91 (4.1%) | 45 (3.0%) | 0.24 |
| Early stent thrombosis | 44 (1.0%) | 6 (1.4%) | 38 (0.9%) | 0.35 | 29 (1.0%) | 15 (0.8%) | 0.52 |
| Late stent thrombosis | 38/4501 (0.9%) | 5/427 (1.2%) | 33/4074 (0.8%) | 0.42 | 23/2752 (0.9%) | 15/1749 (0.9%) | 0.93 |
| Very late stent thrombosis | 54/4187 (2.0%) | 6/387 (1.8%) | 48/3800 (2.0%) | 0.68 | 39/2570 (2.3%) | 15/1617 (1.3%) | 0.17 |
| up to 2 year | 19/4187 (0.5%) | 2/387 (0.5%) | 17/3800 (0.5%) | 0.85 | 13/2570 (0.5%) | 6/1617 (0.4%) | 0.54 |
| 2 year – 3 year | 15/3785 (0.5%) | 2/355 (0.6%) | 13/3430 (0.4%) | 0.62 | 11/2350 (0.5%) | 4/1435 (0.3%) | 0.40 |
| 3 year – 4 year | 12/2879 (0.5%) | 2/285 (0.7%) | 10/2594 (0.5%) | 0.42 | 8/1827 (0.5%) | 4/1052 (0.4%) | 0.87 |
| 4 year – 5 year | 8/1893 (0.6%) | 0/177 (0.0%) | 8/1716 (0.6%) | 0.37 | 7/1233 (0.8%) | 1/660 (0.2%) | 0.20 |
The all-cause mortality rate after ST was 29% and the 30-day mortality rate was 19%, which were higher than that for patients without ST ( Table 4 ). For angiographically confirmed definite early ST, the 30-day mortality rate was significantly higher than that in the no-ST group. In contrast, only 2 deaths occurred within 30 days after definite VLST, and the 5-year all-cause mortality rate for definite VLST was not different.
| 30-day mortality | p Value (vs. No stent thrombosis) | 5-year mortality | p Value (vs. No stent thrombosis) | |
|---|---|---|---|---|
| No stent thrombosis | 193/4584 (4%) | – | 759/4584 (17%) | – |
| Probable/definite stent thrombosis | 26/136 (19%) | <0.001 | 40/136 (29%) | <0.001 |
| Definite stent thrombosis | 9/110 (8%) | 0.054 | 23/110 (21%) | 0.22 |
| Definite early stent thrombosis | 4/29 (14%) | 0.03 | 6/29 (21%) | 0.61 |
| Definite late stent thrombosis | 3/37 (8%) | 0.21 | 8/37 (22%) | 0.41 |
| Definite very late stent thrombosis | 2/44 (5%) | 0.71 | 9/44 (21%) | 0.49 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


