The accuracy of the 12-lead electrocardiogram in detecting coronary artery occlusion is limited. We sought to determine the incidence, distribution, and outcomes of patients who have total occlusion of the culprit artery but present with non–ST-segment elevation myocardial infarction (NSTEMI). The randomized Acute Catheterization and Urgent Intervention Triage Strategy trial enrolled 13,819 patients presenting with non–ST-segment elevation acute coronary syndromes who underwent an early invasive strategy. The present study includes 1,319 patients with baseline biomarker elevation (NSTEMI) and no history of coronary artery bypass graft who underwent percutaneous coronary intervention of a single culprit vessel. We compared the baseline characteristics and outcomes according to whether the culprit vessel was occluded (baseline Thrombolysis In Myocardial Infarction [TIMI] 0 to 1) or patent (TIMI 2 to 3 flow) by angiographic core laboratory assessment. TIMI 0 to 1 flow in the culprit artery was present in 262 of 1,319 (19.9%) patients. The incidence of coronary occlusion was 28.4%, 19.3%, and 12.6% in patients with NSTEMI because of right coronary, left circumflex, and left anterior descending artery disease, respectively. Patients with an occluded culprit artery were more commonly men and had ST-segment deviation ≥1 mm. One-year outcomes, including death (3.5% vs 3.0%, p = 0.68) and myocardial infarction (8.4% vs 9.6%, p = 0.47), did not differ significantly between patients with versus without occluded culprit arteries, respectively. In conclusion, the present study demonstrates that the culprit artery is totally occluded in approximately 1 in 5 patients presenting with NSTEMI and single-vessel disease; however, the presence of total occlusion in NSTEMI was not associated with an incremental hazard of death or reinfarction at 1 year.
The prevalence and significance of complete occlusion of the culprit artery in patients presenting with non–ST-segment elevation myocardial infarction (NSTEMI) may be underestimated. As emergent catheterization and percutaneous coronary intervention (PCI) are believed to be of benefit in patients with total coronary occlusion, failure to manifest ST-segment elevation on the admitting electrocardiography (ECG) may lead to delayed reperfusion in some patients. However, the outcomes of patients with missed occluded culprit arteries (OCAs) in large acute coronary syndrome (ACS) clinical trials have not been examined, but conventional understanding would indicate that complete occlusion would confer a poorer prognosis. Previous studies examining the frequency and significance of an OCA in ACS have arrived at varying conclusions. We, therefore, sought to determine the incidence, distribution, and impact of OCA in patients presenting with NSTEMI from the Acute Catheterization and Urgent Intervention Triage Strategy (ACUITY) trial.
Methods
The study design and methodology of the ACUITY trial have been previously described. In brief, this multicenter, open-label, prospective, randomized trial enrolled 13,819 patients at 450 centers in 17 countries with moderate- and high-risk ACS. Eligible patients presented with unstable angina for ≥10 minutes within the preceding 24 hours with at least one of the following: new ST-segment depression or transient elevation of ≥1 mm, elevated troponin I, troponin T, or creatinine kinase MB isoenzyme, known coronary artery disease, or all 4 other variables in the Thrombolysis In Myocardial Infarction (TIMI) unstable angina risk score. Exclusion criteria were acute STEMI; cardiogenic shock; major bleeding within 2 weeks; thrombocytopenia; calculated creatinine clearance <30 ml/min; recent use of abciximab, warfarin, fondaparinux, fibrinolytics, bivalirudin, or 2 or more doses of low–molecular-weight heparin; or allergy to any study drugs or contrast media. All patients provided written informed consent, and the study was approved by the ethics committee at each center.
Patients were randomized to receive unfractionated heparin or enoxaparin plus a glycoprotein IIb/IIIa inhibitor, bivalirudin plus a glycoprotein IIb/IIIa inhibitor, or bivalirudin alone. All patients enrolled underwent cardiac catheterization within 72 hours of presentation followed by PCI, medical management, or surgical revascularization as per the treating physician. Secondary randomization occurred in the subset of patients receiving glycoprotein IIb/IIIa inhibitors to either administration “upstream” (before angiography) or during PCI alone. All patients received dual antiplatelet therapy for ≥12 months.
In total, 13,819 patients were enrolled in the ACUITY trial. For the present study, we considered 8,153 patients who presented with NSTEMI, defined as having baseline cardiac biomarker elevation in the absence of ST-segment elevation. We excluded 5,867 patients who did not undergo quantitative coronary angiography or were not treated with PCI. We also excluded 862 patients with previous coronary artery bypass surgery. Finally, we excluded 105 patients with multiple target vessels. We compared baseline characteristics and outcomes in patients with an occluded versus patent culprit artery.
An independent core angiographic laboratory (Cardiovascular Research Foundation, New York, New York) identified the culprit artery and classified the degree of TIMI flow. An OCA was defined as a culprit vessel with TIMI 0 to 1 flow, indicating no dye penetration or minimal dye penetration without complete vessel opacification, whereas a patent culprit artery was defined as TIMI 2 to 3 flow, with partial or complete perfusion. Only patients with acute cardiac biomarker elevation were considered for inclusion to avoid enrollment of chronic total occlusion lesions.
The study end point definitions for the ACUITY trial have been previously published. Our primary study end point was major adverse cardiac events (MACE), the composite rate of death, myocardial infarction (reinfarction), or ischemia-driven target vessel revascularization. Clinical end points were collected in-hospital, at 1 month, and 1 year and adjudicated by a blinded clinical events committee.
Categorical variables were expressed as percentages and compared using the chi-square test or Fisher’s exact test. Continuous variables were expressed as a mean ± SD or median with interquartile ranges and were compared using the Student’s t test or Kruskal-Wallis test. One-month and 1-year outcomes were expressed as Kaplan-Meier estimates and compared between groups with log-rank tests.
Results
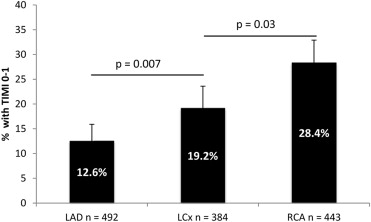
The study population comprised 1,319 patients with NSTEMI without previous coronary artery bypass graft who underwent single-vessel PCI; 262 (20%) presented with an OCA, whereas 1,057 (80%) had a patent culprit vessel. The infarct vessel was occluded in 126 of 443 (28.4%) cases of NSTEMI because of right coronary artery (RCA) disease, in 74 of 384 (19.3%) cases of NSTEMI because of left circumflex (LCx) disease, and in 62 of 492 (12.6%) cases of NSTEMI because of left anterior descending disease ( Figure 1 ). Baseline characteristics according to patency of culprit artery are listed in Table 1 . The cohorts were well matched, varying significantly only in terms of gender and admission ECG. Patients with an OCA were more commonly men and more commonly presented with ST-segment depression ≥1 mm. The TIMI risk score classification was similar in the 2 groups.
| Variable | Occluded Culprit Coronary Artery | P value | |
|---|---|---|---|
| Yes TIMI 0-1 Flow (n = 262) | No TIMI 2-3 Flow (n = 1,057) | ||
| Age (years) | 58 (51–68) | 60 (51–69) | 0.14 |
| Men | 189 (72%) | 687 (65%) | 0.03 |
| Weight (kg) | 87.3 (77–102) | 86.3 (74.5–99) | 0.16 |
| Diabetes mellitus | 70 (27%) | 259 (25%) | 0.48 |
| Insulin-treated | 22 (8%) | 77 (7%) | 0.52 |
| Hypertension (on medication) ∗ | 153 (58%) | 625 (59%) | 0.83 |
| Hyperlipidemia (on medication) † | 108 (42%) | 482 (46%) | 0.21 |
| Current smoker | 112 (43%) | 426 (40%) | 0.44 |
| Previous myocardial infarction | 52 (20%) | 222 (21%) | 0.67 |
| Previous percutaneous coronary intervention | 53 (20%) | 266 (25%) | 0.11 |
| Renal insufficiency | 34 (14%) | 174 (17%) | 0.16 |
| Presentation | |||
| Baseline cardiac biomarker elevation | 262 (100%) | 1,057 (100%) | 1.00 |
| Baseline troponin elevation | 247 (96%) | 989 (95%) | 0.53 |
| ST-segment depression ≥1 mm | 96 (37%) | 274 (26%) | 0.0007 |
| TIMI risk score | |||
| 0-2 | 37 (17%) | 148 (18%) | 1.00 |
| 3-4 | 115 (54%) | 462 (55%) | 0.76 |
| 5-7 | 62 (29%) | 229 (27%) | 0.67 |
∗ Blood pressure >140/90 mm Hg or on medication.
Angiographic characteristics are presented in Table 2 . Patients with an OCA had shorter durations from admission to PCI. Patients with an OCA had a lower left ventricular ejection fraction and were more likely to have thrombotic culprit lesions and angiographically visible collateral vessels. Periprocedural medication regimens were comparable between groups, but patients with a patent culprit artery were more likely to receive a drug-eluting stent.
| Variable | Occluded Culprit Coronary Artery | P value | |
|---|---|---|---|
| Yes TIMI 0-1 Flow (n = 262) | No TIMI 2-3 Flow (n = 1,057) | ||
| Culprit coronary artery | |||
| Left anterior descending | 62 (24%) | 430 (41%) | <0.0001 |
| Left circumflex | 74 (28%) | 310 (29%) | 0.76 |
| Right | 126 (48%) | 317 (30%) | <0.0001 |
| Stent type | |||
| Drug-eluting | 195 (74%) | 883 (84%) | 0.0009 |
| Bare metal | 41 (16%) | 146 (14%) | 0.43 |
| Both | 12 (4.6%) | 40 (3.8%) | 0.59 |
| Treatment timing (hours) | |||
| Admission to randomization | 8.8 ± 10.4 | 11.0 ± 15.0 | 0.01 |
| Randomization to study drug | 1.5 ± 2.7 | 1.3 ± 2.4 | 0.23 |
| Study drug to angiography | 7.7 ± 12.4 | 10.6 ± 17.7 | 0.002 |
| Ejection fraction (%) | 62.5 [53.9, 70.1] | 65.5 [57.3, 71.7] | 0.01 |
| Lesion morphology | |||
| Thrombus | 130/275 (47%) | 155/1228 (13%) | <0.0001 |
| Spasm | 9/274 (3.3%) | 30/1228 (2.4%) | 0.40 |
| Blush | |||
| 0-1 | 58 (24%) | 28 (2.8%) | <0.0001 |
| 2 | 20 (8.1%) | 137 (14%) | 0.02 |
| 3 | 168 (68%) | 826 (83%) | <0.0001 |
| Calcium | |||
| None/mild | 205 (80%) | 835 (80%) | 0.93 |
| Moderate | 45 (17%) | 180 (17%) | 0.93 |
| Severe | 8 (3.1%) | 32 (3.1%) | 1.00 |
| Collaterals present | 48 (18%) | 37 (3.5%) | <0.0001 |
| Glycoprotein IIb/IIIa inhibitor use | |||
| Pre-angiography | 96 (37%) | 334 (32%) | 0.12 |
| Eptifibatide | 93 (36%) | 323 (31%) | 0.14 |
| Tirofiban | 3 (1.1%) | 10 (0.9%) | 0.73 |
| Abciximab | 0 (0%) | 1 (0.1%) | 1.00 |
| During PCI | 194 (74%) | 721 (68%) | 0.07 |
| Eptifibatide | 172 (66%) | 662 (63%) | 0.39 |
| Tirofiban | 4 (1.5%) | 14 (1.3%) | 0.77 |
| Abciximab | 18 (6.9%) | 45 (4.3%) | 0.10 |
The rates of adjudicated adverse events at 1 month and 1 year are presented in Table 3 and Figure 2 . The rate of 1-year MACE did not differ in patients with and without an OCA (18.4% vs 16.4%, respectively, p = 0.50). The rates of mortality, reinfarction, and stent thrombosis did not differ significantly between groups. Patients with an OCA were more likely to require target vessel revascularization within 1 month, although this difference did not persist at 1-year follow-up. We also examined whether the importance of culprit artery occlusion varied according to the specific vessel affected ( Table 4 and Figure 3 ). The 1-year rate of MACE and its individual components were similar in each vessel according to whether the culprit coronary artery was or was not initially occluded.
| Variable | Occluded Culprit Coronary Artery | p Value | |
|---|---|---|---|
| Yes TIMI 0-1 Flow (n = 262) | No TIMI 2-3 Flow (n = 1,057) | ||
| 30-day outcomes | |||
| Major adverse cardiac events | 26 (10%) | 101 (9.6%) | 0.87 |
| Death | 2 (0.8%) | 12 (1.1%) | 0.60 |
| Cardiac | 2 (0.8%) | 10 (1.0%) | 0.78 |
| Non-cardiac | 0 (0%) | 2 (0.2%) | 0.48 |
| Myocardial infarction | 12 (4.6%) | 76 (7.2%) | 0.13 |
| Target vessel revascularization | 13 (5.0%) | 20 (1.9%) | 0.004 |
| Stent thrombosis | 1 (0.4%) | 15 (1.4%) | 0.17 |
| TIMI major bleeding (non-CABG) | 18 (3.1%) | 7 (0.7%) | 0.001 |
| 1-Year outcomes | |||
| Major adverse cardiac events | 47 (18.4%) | 170 (16.4%) | 0.50 |
| Death | 9 (3.5%) | 31 (3.0%) | 0.68 |
| Cardiac | 5 (1.9%) | 19 (1.8%) | 0.91 |
| Non-cardiac | 3 (1.2%) | 11 (1.1%) | 0.89 |
| Myocardial infarction | 21 (8.4%) | 99 (9.6%) | 0.47 |
| Target vessel revascularization | 24 (9.5%) | 66 (6.5%) | 0.08 |
| Stent thrombosis | 2 (0.8%) | 19 (1.8%) | 0.23 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


