Fig 1.
Poorly differentiated adenocarcinoma. Computed tomography (CT) shows a nodule with a spiculated margin in the right upper lobe. Note that spiculation is typical for primary lung malignancy. At resection pleural invasion was present
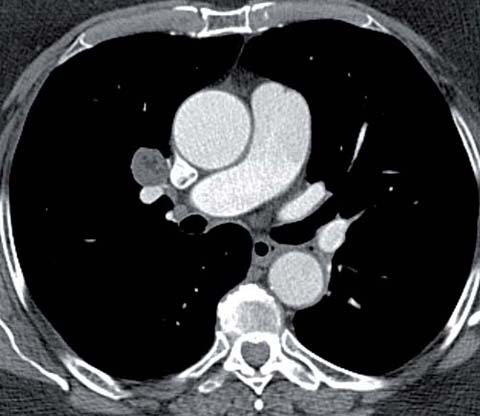
Fig 2.
Hamartoma in a 76-year-old woman. CT shows a 2-cm centrally located nodule in the right upper lobe containing focal low attenuation (−50 HU), consistent with a hamartoma
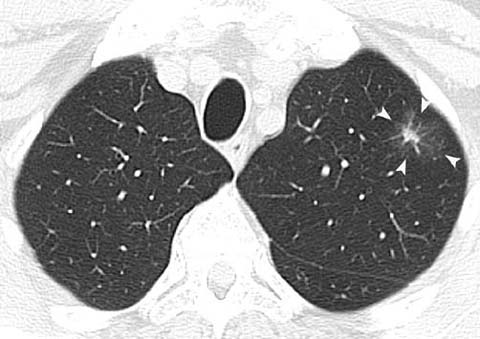
Fig 3.
Invasive adenocarcinoma in a 79-year-old man. CT shows a partly solid nodule in the left upper lobe with an 8-mm solid component (arrowheads). Note that the presence of a >5-mm soft-tissue component on CT makes the likelihood of malignancy high
Significant changes in the pathologic classification of lung adenocarcinoma were proposed in 2011 [16]. The new adenocarcinoma classification now includes the terms adenocarcinoma in situ (AIS), a preinvasive lesion, minimally invasive adenocarcinoma (MIA), and invasive adenocarcinoma [16]. AIS was formerly classified as a bronchioloalveolar cell carcinoma and is a small (≼3 cm), localized adenocarcinoma that has no stromal, vascular, or pleural invasion and demonstrates lepidic growth [16]. MIA is a solitary adenocarcinoma (≼3 cm) with ≼5-mm invasion in any one focus and a predominantly lepidic pattern of growth [16]. MIA is excluded if the tumor invades the lymphatics, blood vessels, or pleura or contains necrosis. Invasive adenocarcinomas are composed of a complex heterogeneous mixture of histologic subtypes and are classified according to the most predominant one (lepidic, acinar, papillary, micropapillary, or mostly solid patterns) [16]. For mucinous malignancies, the term invasive mucinous adenocarcinoma has replaced mucinous bronchioloalveolar cell carcinoma. These tumors differ from mucinous AIS and MIA by a size >3 cm and/or showing invasion >0.5 cm and/or multiple nodules, or the lack of a circumscribed border with miliary spread into adjacent lung parenchyma. Invasive mucinous adenocarcinomas typically manifest as solid nodules or consolidative opacities.
Positron Emission Tomography (PET) Findings
The conventional radiologic assessment of an SPN is complemented by the use of PET and the radiopharmaceutical 18F-2-deoxy-D-glucose (FDG), a D-glucose analog labeled with fluorine-18. The reported sensitivity and specificity of PET for malignant pulmonary lesions are 97% and 78%, respectively. The spatial resolution of PET scanners is in the range of 6 mm; therefore, smaller lesions should not be evaluated with PET. When FDG uptake by a solid nodule ≽ 1 cm in diameter is low, the likelihood of malignancy is generally considered to be low. However, in a study of 360 patients with lung nodules evaluated by FDG-PET, 43 patients had solid nodules with a standardized uptake value (SUV) <2.5, 16 of which were malignant [17]. False-negative results for malignancy include slow-growing cancers, especially adenocarcinomas with subsolid morphology and carcinoid tumors (Fig. 4). Nomori et al. reported that nine of ten well-differentiated adenocarcinomas manifesting as ground glass nodular opacities were falsely negative on FDG-PET [18]. The sensitivity (10%) and specificity (20%) for ground glass opacities in that study were significantly lower than for solid nodules (90% and 71%, respectively). False-positive results are most commonly caused by infections and inflammatory processes, including Wegener’s granulomatosis, sarcoidosis, organizing pneumonia, amyloid and rheumatoid nodules.
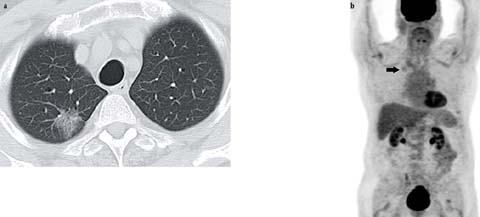
Fig 4 a, b.
Adenocarcinoma in situ in an 89-year-old man. CT shows a 3-cm pure ground glass nodular opacity in the right upper lobe. b Whole-body positron emission tomography (PET) maximum intensity projection image shows low-grade 18F-2-deoxy-D-glucose (FDG) uptake in the nodule (arrow). Note that adenocarcinomas manifesting as ground glass nodular opacities are frequently falsely negative on FDG-PET
Pulmonary nodules can be characterized according to their growth, morphology, and metabolic activity. Standard imaging protocols based on the pretest probability (risk) should be used to rule out malignancy.
Guidelines for the Evaluation of an Incidentally Detected Pulmonary Nodule
The Fleischner Society guidelines for the evaluation of an incidentally discovered solid nodule in an adult patient integrate lesion morphology, growth rate, patient age, and smoking history (see below). In addition, to complement the recommendations for incidentally detected solid nodules, the Society recently published recommendations specifically aimed at the management of ground glass and partly solid nodules (see below) [19, 20]. Importantly, for both, risk factors such as smoking history, family history of lung cancer, or exposure to carcinogenic agents are not considered in the current guidelines due to a lack of sufficient data. Other issues to be aware of are that a slight temporary decrease in size can be seen with adenocarcinomas manifesting as ground glass or partly solid nodules, due to fibrosis or atelectasis, and enlargement and/or increasing attenuation with or without the new appearance of a solid component during follow up should be managed with a high degree of suspicion [20].
Recommendations for the Management of Incidentally Detected Solid Nodules
The Fleischner Society guidelines [19] are summarized in the following.
Low-Risk Populations (Little or No History of Smoking, and No Other Risk Factors)
1.
Nodules ≼4 mm have a very small likelihood of malignancy such that re-assessment is not necessary
2.
Nodules >4-mm but ≼6-mm should be re-assessed using CT at 12 months; if stable, no further evaluation is required. The exception is the non-solid or partially solid nodule, for which re-assessment may need to be continued to exclude the risk of an indolent adenocarcinoma.
3.
Nodules >6 mm but ≼8 mm should be re-assessed using CT at 6–12 months and, if stable, again at 18–24 months.
4.
Nodules >8 mm should either be re-assessed using CT at 3, 9, and 24 months, to determine their size stability, or further evaluated with contrast-enhanced CT, CT-PET, biopsy, or resection.
High-Risk Populations (History of Smoking, or Other Exposure or Risk Factor)
1.
Nodules ≼4 mm should be re-assessed at 12 months; if stable, no further evaluation is required. The exception is the non-solid or partially solid nodule, for which re-assessment may need to be continued to exclude the risk of an indolent adenocarcinoma.
2.
Nodules >4 mm but ≼6 mm should be re-assessed using CT at 6–12 months and, if stable, again at 18–24 months.
3.
Nodules >6 mm but ≼8 mm should be re-assessed using CT at 3–6 months and, if stable, again at 9–12 months and 24 months.
4.
Nodules >8 mm should either be re-assessed using CT at 3, 9, and 24 months to assess stability or a contrast-enhanced CT, CT-PET, biopsy, or resection should be performed.
Recommendations for the Management of Incidentally Detected Solitary Ground Glass and Part Solid Nodules
The Fleischner Society guidelines [20] are summarized in the following.
1.
Solitary pure ground glass nodules ≼5 mm require no CT follow-up.
2.
Nodules >5 mm should be initially followed-up at 3 months using CT to confirm persistence, followed by annual surveillance CT for a minimum of 3 years.
3.
Solitary, partly solid nodules should be initially followed-up at 3 months using CT to confirm persistence. If persistent, with a solid component <5 mm, yearly surveillance CT should be performed for a minimum of 3 years. If persistent with a solid component ≽5 mm, then biopsy or surgical resection is recommended. PET/CT should be considered for partly solid nodules >10 mm.
Recommendations for the Management of Incidentally Detected Multiple Ground Glass and Part Solid Nodules
1.
Pure ground glass nodules ≼5 mm should be followed-up using CT at 2 and 4 years.
2.
Pure ground glass nodules >5 mm without a dominant lesion(s) should be initially followed-up using CT at 3 months to confirm persistence, after which annual surveillance CT for a minimum of 3 years should be performed.
3.
A dominant nodule(s) with a partly solid or solid component should be initially followed-up at 3 months using CT to confirm persistence. If persistent, biopsy or surgical resection is recommended, especially for lesions with a solid component >5 mm.
Staging of Lung Cancer
Staging, a standardized anatomic description of disease extent, is determined both clinically and pathologically and guides appropriate treatment. In general, the clinical stage underestimates the extent of disease compared with the pathologic stage. Radiologic imaging is an essential component of clinical staging and allows the assessment of disease manifestations that are important for surgical, oncological, and radiation therapy planning, including size of the primary tumor, its location and relationship to normal anatomic structures in the thorax, and the presence of nodal and or metastatic disease. The 7th edition of the tumor node metastasis (TNM) staging system allows a standardized definition of stage and consequently, indicates the most appropriate treatment. While useful in ascertaining advanced disease, chest radiography is limited in accurately determining TNM descriptors in patients with potentially resectable disease, which typically requires imaging with CT and/or magnetic resonance imaging (MRI) and/or PET/CT. A contrast-enhanced CT of the chest is recommended for the evaluation of all patients with known or suspected primary lung cancer [21]. CT accurately assesses most characteristics of the primary tumor (T descriptor), including size and location, but loco-egional invasion can be difficult to determine. CT is also useful in detecting nodal metastasis and determining the absence or presence of intra- and extrathoracic metastatic disease, including contralateral lung nodule(s), pleural and pericardial nodule(s) and effusions, bone metastases, and adrenal nodules/masses. CT of the chest alone is sufficient for the staging of patients with pure ground glass nodules and an otherwise normal study, and for patients with peripheral stage IA disease [21]. Otherwise, further imaging with FDG-PET is recommended for patients eligible for curative treatment. FDG-PET/CT has an increasing role in staging, particularly for detecting nodal (N descriptor) and distant (M descriptor) metastasis. When PET is unavailable or cannot be performed a contrast-enhanced CT of the abdomen is recommended [21].
T Descriptor
The T descriptor defines the size, location, and extent of the primary tumor and is based on differences in survival (Fig. 5). However, although a T4 descriptor generally precludes resection, tumors with cardiac, tracheal, and vertebral-body invasion are designated in the 7th edition staging system as being potentially resectable in the absence of N2 and or N3 disease.

Fig 5.
Descriptions of malignant lung tumors, nodes, and metastases for classification purposes. Reprinted with permission courtesy of the International Association for the Study of Lung Cancer. Copyright 2009 IASLC
The following parameters must be analyzed regarding T descriptor determination: (1) size, (2) location, (3) extension.
1.
Tumor size is a determinant of the T descriptor and essential to define surgical and radiotherapy strategies as well as to evaluate the response to treatment. In the 7th edition of the TNM classification, size is a significant parameter related to survival. T1 is subclassified as T1a (<2 cm) or T1b (>2 cm to <3 cm); T2 is subclassified as T2a (>3 cm to <5 cm or T2 by other factor and <5 cm) or T2b (>5 cm to <7 cm); tumors >7 cm are classified as T3. Size is usually measured on CT or MRI. On CT, lung window settings should be used as mediastinal windowing may underestimate the size.
2.
Tumor location is important in that it provides information to surgeons and radiation oncologists that can affect therapeutic management. For instance, centrally located tumors close to the spinal cord impose radiation dose-volume constraints, and determining tumor margins is important as they can affect radiotherapy delivery. This is especially important with the increasing use of conformal radiation therapy, in which multiple radiation beams are used to generate dose distributions that conform tightly to target volumes. The relation of the tumor to a fissure must be reported, as it may imply a change in surgical technique (pneumonectomy instead of lobectomy) when there is evidence that the lesion crosses the fissure (Fig. 6). The proximity to a main pulmonary artery can also involve a change in surgical approach.
3.
Tumor extension can be divided into local and distant. Local extension is included in the T descriptor, whereas distant metastases relate to the M descriptor. In the TNM staging system, additional pulmonary nodules in the same lobe are classified as T3, nodule/s in other ipsilateral lobes are T4 and nodule/s in the contralateral lung are M1a.The determination of the degree of pleural, chest-wall, and mediastinal invasion, as well as central airways, pulmonary veins, and artery involvement is important not only to radiation oncologists but also to surgeons evaluating the tumor for resectability. For instance, pulmonary artery involvement may require a pneumonectomy rather than a lobectomy in order to obtain clear surgical margins. Additionally, involvement of the origin of the lobar bronchus or main bronchus may require a sleeve resection or pneumonectomy. Thoracic wall invasion does not preclude surgery but must be reported to the surgeon to ensure appropriate surgical resection, typically en bloc resection of the chest wall.
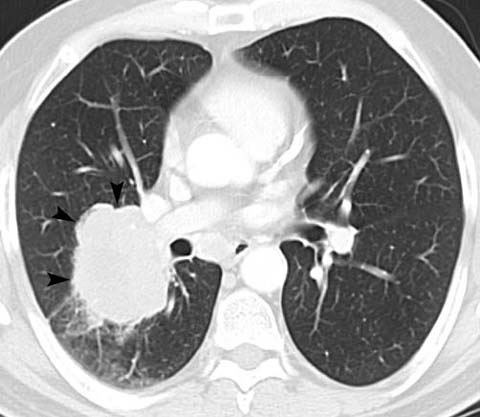
Fig 6.
Poorly differentiated adenocarcinoma in a 60-year-old man. CT shows a lobular mass in the right lower lobe with extension through the major fissure (arrowheads). The patient was treated definitively with concurrent chemoradiation
Role of Imaging in T Descriptor Determination
CT and MRI are useful in determining gross chest wall or mediastinal invasion (Fig. 7). However, limited loco-regional invasion is difficult to differentiate from abutment. MRI can be used to assess myocardial invasion and to evaluate superior sulcus tumors for involvement of the brachial plexus, regional vasculature, and adjacent spine and vertebra (Fig. 8). This is important since involvement of the brachial plexus roots or trunks superior to the T1 nerve root, invasion of the trachea or esophagus, and >50% invasion of a vertebral body are absolute contraindications to surgical resection [22, 23].
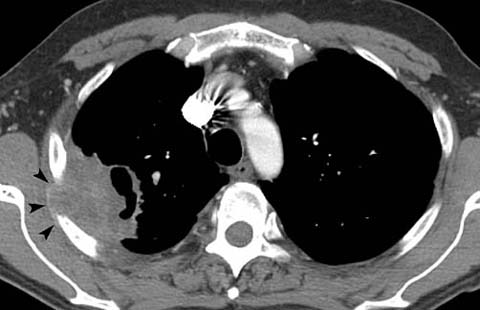
Fig 7.
Non-small-cell lung cancer in a 50-year-old man with chest-wall pain. CT shows a large cavitary mass in the right upper lobe with invasion of the chest wall (arrowheads). Note that chest wall invasion does not preclude surgery but usually changes the resection from a lobectomy to an en bloc resection of the lobe and chest wall
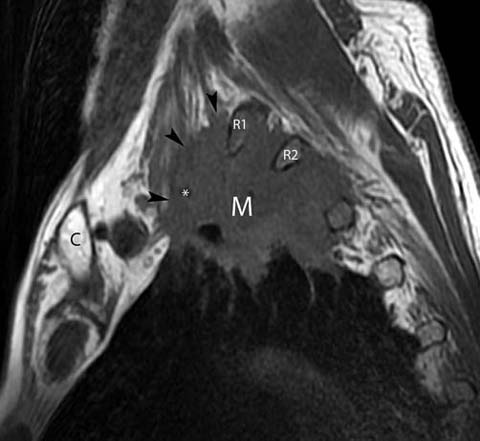
Fig 8.
Superior sulcus tumor in a 66-year-old man presenting with numbness of the upper arm extending to the elbow. Sagittal T1-weighted magnetic resonance image shows a mass (M) in the left upper lobe. The mass extends above the first rib and involves the C7 and C8 nerve roots of the brachial plexus (arrowheads). There is also extension of the mass into the soft tissues of the thorax posteriorly into the T1/2 and T2/3 intevertebral foramina. The mass encases the subclavian artery (*). Note that involvement of the brachial plexus roots superior to the T1 nerve root is an absolute contraindication to surgical resection. C clavicle, R1 first rib, R2 second rib
Tumor size, location, and extension are the fundamental T descriptors. The radiologist must have a thorough knowledge of the anatomic implications of the T descriptors in relation to surgery, radiotherapy, and chemotherapy. CT and MRI play fundamental roles in T descriptor determination. PET/CT is an additional tool in specific situations.
N Descriptor
The N descriptor, which specifies the presence and location of nodal metastatic disease, has a significant effect on management (Fig. 5). N descriptors are designated according to lymph node maps in which the nodal stations are numbered based on relationships to anatomic structures [24, 25]. For the 7th TNM edition, a standardized nodal map with seven node zones, created by unifying the Mountain/Dressler-ATS (MD-ATS) node map and the Japanese Naruke map, is used. The International Association for the Study of Lung Cancer (IASLC) nodal map designates the oncologic midline of the superior mediastinum to correspond with the left lateral border of the trachea, such that all nodes anterior to the trachea are grouped with right paratracheal nodes (Fig. 9). Surgical resection and the potential use of adjuvant therapy depend on the N descriptor, such that its accurate determination is important. Ipsilateral peribronchial, or hilar (N1) nodes are usually resectable while mediastinal nodes have a major influence on resectability. Specifically, ipsilateral mediastinal (including subcarinal) nodal metastasis (N2) can be resectable (usually after induction chemotherapy), while contralateral mediastinal and scalene or supraclavicular disease (N3) is not. To potentially improve patient management, data are currently being collected based on grouping nodal stations together in six zones within the current N1 and N2 patient subsets for further evaluation [26].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


