Large thrombus burden negatively affects the results of percutaneous coronary intervention (PCI) for acute ST-segment elevation myocardial infarction (STEMI). We investigated the impact of thrombus burden in patients with STEMI undergoing primary PCI with the mesh-covered MGuard stent (InspireMD Ltd., Tel Aviv, Israel) versus a control bare-metal or drug-eluting stent. In 433 patients with STEMI randomized to the MGuard stent versus a control stent, angiographically visible thrombus was identified in 383 patients (88.5%), with median thrombus area 30.15 mm 2 (22.70, 41.93). Lesions with large thrombus (area > median) were treated with more frequent use of manual aspiration (80.8% vs 65.8%, p = 0.0009) and longer (22.1 ± 5.9 vs 19.4 ± 5.4 mm, p <0.0001) and larger (3.46 ± 0.40 vs 3.29 ± 0.36 mm, p <0.0001) stents. PCI of lesions with large thrombus burden had more thrombotic complications (30.6% vs 15.9%, p = 0.0007) and reduced angiographic success (80.3% vs 91.1%, p = 0.003). In large thrombus lesions, the MGuard stent was more effective than control stents in achieving Thrombolysis In Myocardial Infarction-3 flow (87.9% vs 74.5%, p = 0.02) and tended to result in less slow flow or no reflow (8.8% vs 17.6%, p = 0.07). ST-segment resolution was improved with the MGuard, and clinical outcomes were favorable in both stent groups, regardless of thrombus burden. In conclusion, reperfusion success is reduced after primary PCI in lesions with large thrombus burden, an outcome that may be modified by the MGuard stent.
Mechanical reperfusion is the standard of care for patients with ST-segment elevation myocardial infarction (STEMI). The amount or “burden” of thrombus in patients with STEMI undergoing primary percutaneous coronary intervention (PCI) has been identified as a major determinant of outcomes, having been associated with reduced procedural success and worse early and late event-free survival. The MGuard embolic protection stent (InspireMD Ltd., Tel Aviv, Israel) is a mesh-covered metallic stent designed to trap and exclude friable thrombotic and atheromatous material, which has shown promise in reducing distal embolization and thrombotic complications during PCI. Whether the MGuard stent is particularly effective in patients with large thrombus burden is unknown. We therefore investigated the impact of thrombus burden on angiographic and clinical outcomes in patients with STEMI undergoing primary PCI with the MGuard stent versus conventional stents in the MGuard for Acute ST Elevation Reperfusion (MASTER) trial.
Methods
The design and principal outcomes of the MASTER trial have been previously reported. In brief, 433 patients with STEMI of ≤12 hours duration and a single de novo lesion ≤33 mm in length in a native coronary vessel 3.0 to 4.0 mm in diameter were randomized 1:1 to PCI with the MGuard stent versus any commercially available bare-metal or drug-eluting stent. All patients were treated with aspirin (75 to 162 mg/day) indefinitely and a P2Y 12 inhibitor for 1 year. For the purpose of the current analysis, only patients with an angiographically evident thrombus (as assessed by independent angiographic core laboratory analysis) were included. The performance of manual thrombus aspiration and/or predilatation was left to operator discretion.
Baseline and final quantitative coronary angiography analyses were performed by technicians blinded to randomization with standard method using validated software for quantitative analysis (QAngio XA version 7.3, Medis, Leiden, the Netherlands). Anterograde coronary blood flow was determined according to the Thrombolysis In Myocardial Infarction (TIMI) classification. Corrected TIMI frame count was assessed as previously described. Myocardial blush grade (MBG) was graded according to previously described method. Thrombus was defined as the presence of an intraluminal radiolucent or “negative” contrast density, with or without defined borders, usually separated from the adjacent vessel wall, with or without contrast staining. Thrombus was also classified into 4 types as follows: globular, filling defect, haziness, or total occlusion. During PCI, intraprocedural thrombotic events were defined as the development of new or increasing thrombus, abrupt vessel closure, no reflow, slow reflow, distal embolization, side branch closure, or intraprocedural stent thrombosis occurring at any time during the procedure, as reported by the core laboratory after frame-by-frame review. Thrombus area was measured as shown in Figure 1 using the following formula: reference diameter (mm) × lesion (“thrombus”) length (mm) × diameter stenosis (0 to 1.0). In case of a total occlusion, thrombus length was determined after flow restoration within the target vessel.
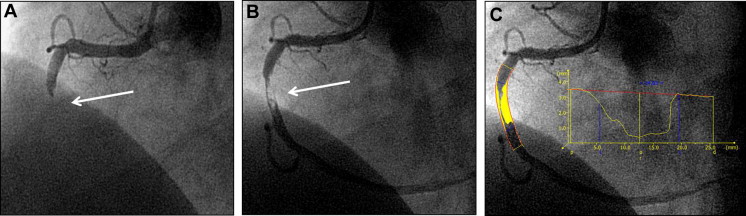
Prespecified efficacy endpoints included (1) complete ST-segment resolution (≥70% reduction in the extent of ST-segment elevation in the summed 12-lead electrocardiogram from baseline to postprocedure); (2) final TIMI flow and corrected TIMI frame count in the infarct-related coronary artery; and (3) postprocedure MBG. All electrocardiographic analyses were determined by a blinded, independent electrocardiographic core laboratory as previously described. Major adverse cardiovascular events were defined as the composite of cardiac death, reinfarction, or ischemia-driven target lesion revascularization. Stent thrombosis was defined according to the Academic Research Consortium’s criteria. Adverse clinical events were adjudicated by an independent clinical events committee blinded to treatment assignment.
Categorical variables are presented as counts and frequencies and were compared with the chi-square or Fisher’s exact test. Continuous variables are presented as mean ± SD and were compared with the Student t test. Analyses were performed in subgroups according to whether the infarct lesion had small thrombus burden (≤median measured thrombus area) or large thrombus burden (>median measured thrombus area) as determined by the angiographic core laboratory. Interaction testing was performed by logistic regression. All statistical analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, North Carolina). A p value <0.05 was considered statistically significant.
Results
Of the 433 patients enrolled in the MASTER trial, 383 patients (88.5%) had infarct lesions with angiographically evident thrombus, with median area of 30.15 mm 2 (22.70, 41.93). These patients were divided into 2 groups according to thrombus size, large thrombus (mean thrombus area 47 ± 18 mm 2 ) and small thrombus (mean thrombus area 22 ± 6 mm 2 ).
Baseline clinical characteristics are listed in Table 1 . Apart from previous myocardial infarction, there were no significant clinical differences between the groups with large and small thrombus burden. However, those with large thrombus had more complex lesion morphology, longer lesion lengths, and larger reference vessel diameters ( Table 2 ). Manual thrombus aspiration was more frequently performed in the large thrombus group, and this group was treated with longer and larger diameter stents ( Table 3 ). Intraprocedural thrombotic events occurred more frequently in patients with large versus small thrombus (30.6% vs 15.9%, p = 0.0007), and stenting lesions with large thrombus was associated with lower rates of final TIMI flow grade 3 and MBG 2/3 ( Figure 2 ), and greater TIMI frame counts ( Table 3 ). There were no significant differences in 30-day event rates in patients with large versus small thrombus burden, including major adverse cardiovascular events (3.1% [n = 6] vs 1.1% [n = 2], p = 0.16), cardiac death (1.6% [3] vs 0.5% [1], p = 0.33), and definite or probable stent thrombosis (1.6% [3] vs 0.5% [1], p = 0.32).
| Variable | All Patients | Small Thrombus | Large Thrombus | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Small Thrombus (n = 190) | Large Thrombus (n = 193) | p Value | MGuard (n = 97) | Control (n = 93) | p Value | MGuard (n = 91) | Control (n = 102) | p Value | |
| Age (years) | 58.8 ± 11.6 | 59.6 ± 10.7 | 0.69 | 58.2 ± 10.6 | 59.4 ± 12.5 | 0.48 | 60.2 ± 10.5 | 59.1 ± 10.8 | 0.47 |
| Women | 50 (26%) | 38 (20%) | 0.12 | 25 (26%) | 25 (27%) | 0.86 | 18 (20%) | 20 (20%) | 0.99 |
| Diabetes mellitus | 26 (14%) | 32 (17%) | 0.43 | 10 (10%) | 16 (17%) | 0.17 | 13 (14%) | 19 (19%) | 0.42 |
| Hypertension, medically-treated | 82 (44%) | 87 (46%) | 0.67 | 39 (40%) | 43 (48%) | 0.30 | 38 (43%) | 49 (49%) | 0.39 |
| Dyslipidemia, medically-treated | 50 (27%) | 52 (28%) | 0.77 | 26 (27%) | 24 (26%) | 0.91 | 23 (26%) | 29 (30%) | 0.63 |
| Smoker (current) | 98 (52%) | 100 (53%) | 0.92 | 53 (56%) | 45 (48%) | 0.31 | 51 (57%) | 49 (49%) | 0.29 |
| Prior angina pectoris | 26 (14%) | 20 (10%) | 0.32 | 10 (10%) | 16 (17%) | 0.17 | 8 (9%) | 12 (12%) | 0.50 |
| Prior myocardial infarction | 7 (4%) | 18 (9%) | 0.03 | 1 (1%) | 6 (7%) | 0.06 | 5 (6%) | 13 (13%) | 0.08 |
| Prior percutaneous coronary intervention | 5 (3%) | 13 (7%) | 0.058 | 1 (1%) | 4 (4%) | 0.20 | 5 (6%) | 8 (8%) | 0.52 |
| Symptom to device time (minutes) | 257.5 ± 147.3 | 266.3 ± 164.8 | 0.58 | 251.4 ± 133.7 | 263.9 ± 160.9 | 0.56 | 244.7 ± 145.5 | 285.6 ± 178.8 | 0.08 |
| Variable | All Patients | Small Thrombus | Large Thrombus | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Small Thrombus (n = 190) | Large Thrombus (n = 193) | p Value | MGuard (n = 97) | Control (n = 93) | p Value | MGuard (n = 91) | Control (n = 102) | p Value | |
| Target coronary artery | |||||||||
| Left anterior descending | 72 (38%) | 71 (37%) | 0.82 | 35 (36%) | 37 (40%) | 0.60 | 34 (37%) | 37 (36%) | 0.88 |
| Left circumflex | 19 (10%) | 15 (8%) | 0.44 | 11 (11%) | 8 (9%) | 0.53 | 7 (8%) | 8 (8%) | 0.97 |
| Right | 99 (52%) | 107 (55%) | 0.51 | 51 (53%) | 48 (52%) | 0.89 | 50 (55%) | 57 (56%) | 0.90 |
| Thrombus type | |||||||||
| Globular | 34 (18%) | 44 (23%) | 0.23 | 20 (21%) | 14 (15%) | 0.32 | 20 (22%) | 24 (24%) | 0.80 |
| Filling defect | 22 (12%) | 21 (11%) | 0.83 | 14 (14%) | 8 (9%) | 0.21 | 9 (10%) | 12 (12%) | 0.68 |
| Haziness | 22 (12%) | 12 (6%) | 0.07 | 12 (12%) | 10 (11%) | 0.73 | 8 (9%) | 4 (4%) | 0.16 |
| Total occlusion | 112 (59%) | 116 (60%) | 0.82 | 51 (53%) | 61 (66%) | 0.07 | 54 (59%) | 62 (61%) | 0.84 |
| Thrombus area (mm 2 ) | 21.92 ± 5.69 | 47.05 ± 17.76 | <0.0001 | 21.50 ± 5.95 | 22.36 ± 5.39 | 0.30 | 48.28 ± 20.07 | 45.94 ± 15.44 | 0.37 |
| Aneurysm | 0 (0%) | 6 (3%) | 0.03 | 0 (0%) | 0 (0%) | – | 3 (3%) | 3 (3%) | >0.99 |
| Lesion class C ∗ | 27 (14%) | 44 (23%) | 0.03 | 15 (16%) | 12 (13%) | 0.61 | 25 (28%) | 19 (19%) | 0.14 |
| TIMI flow grade | |||||||||
| 0/1 | 143 (75%) | 154 (80%) | 0.29 | 65 (67%) | 78 (84%) | 0.007 | 74 (81%) | 80 (78%) | 0.62 |
| 2 | 26 (14%) | 22 (11%) | 0.50 | 19 (20%) | 7 (8%) | 0.02 | 10 (11%) | 12 (12%) | 0.87 |
| 3 | 21 (11%) | 17 (9%) | 0.46 | 13 (13%) | 8 (9%) | 0.29 | 7 (8%) | 10 (10%) | 0.61 |
| Myocardial blush grade | |||||||||
| 0/1 | 163 (87%) | 171 (89%) | 0.67 | 81 (84%) | 82 (90%) | 0.24 | 81 (89%) | 90 (88%) | 0.87 |
| 2/3 | 24 (13%) | 22 (11%) | 0.67 | 15 (16%) | 9 (10%) | 0.24 | 10 (11%) | 12 (12%) | 0.87 |
| Quantitative measures | |||||||||
| Lesion length (mm) | 13.3 ± 5.6 | 16.8 ± 8.0 | <0.0001 | 13.9 ± 6.2 | 12.7 ± 4.8 | 0.12 | 17.3 ± 9.7 | 16.3 ± 6.1 | 0.43 |
| Reference vessel diameter (mm) | 3.05 ± 0.38 | 3.28 ± 0.40 | <0.0001 | 3.05 ± 0.36 | 3.05 ± 0.39 | 0.97 | 3.29 ± 0.42 | 3.27 ± 0.40 | 0.72 |
∗ According to the American College of Cardiology/American Heart Association classification–the following variables were considered for type C lesion: diffuse lesion (>20 mm in length); excessive tortuosity of proximal segment (target lesion distal to three bends ≥ 75 degrees); extremely angulated, >90 degrees; and inability to protect major side branch.
| Variable | All Patients | Small Thrombus | Large Thrombus | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Small Thrombus (n = 190) | Large Thrombus (n = 193) | p Value | MGuard (n = 97) | Control (n = 93) | p Value | MGuard (n = 91) | Control (n = 102) | p Value | |
| Glycoprotein IIb/IIIa inhibitor use | 157 (83%) | 168 (87%) | 0.23 | 84 (87%) | 73 (79%) | 0.14 | 75 (82%) | 93 (91%) | 0.07 |
| Unfractioned heparin | 182 (96%) | 189 (98%) | 0.23 | 94 (97%) | 88 (95%) | 0.49 | 88 (97%) | 101 (99%) | 0.34 |
| Bivalirudin | 25 (13%) | 18 (9%) | 0.24 | 8 (8%) | 17 (18%) | 0.04 | 11 (12%) | 7 (7%) | 0.21 |
| Aspiration performed | 125 (66%) | 156 (81%) | 0.0009 | 62 (64%) | 63 (68%) | 0.58 | 78 (86%) | 78 (77%) | 0.10 |
| Balloon predilatation | 91 (48%) | 88 (46%) | 0.65 | 49 (51%) | 42 (45%) | 0.46 | 42 (46%) | 46 (45%) | 0.88 |
| Type of stent implanted | |||||||||
| MGuard | 94 (50%) | 87 (45%) | 0.36 | 93 (97%) | 1 (1%) | <0.0001 | 87 (96%) | 0 (0%) | <0.0001 |
| Bare metal stent | 56 (30%) | 64 (33%) | 0.46 | 2 (2%) | 54 (58%) | <0.0001 | 1 (1%) | 63 (62%) | <0.0001 |
| Drug-eluting stent | 39 (21%) | 42 (22%) | 0.79 | 1 (1%) | 38 (41%) | <0.0001 | 3 (3%) | 39 (38%) | <0.0001 |
| >1 stent implanted | 22 (12%) | 27 (14%) | 0.48 | 16 (17%) | 6 (7%) | 0.03 | 11 (12%) | 16 (16%) | 0.47 |
| Nominal stent length (mm) | 19.4 ± 5.4 | 22.1 ± 5.9 | <0.0001 | 19.8 ± 5.5 | 19.0 ± 5.3 | 0.34 | 22.5 ± 5.6 | 21.8 ± 6.1 | 0.47 |
| Nominal stent diameter (mm) | 3.29 ± 0.36 | 3.46 ± 0.40 | <0.0001 | 3.29 ± 0.33 | 3.28 ± 0.33 | 0.90 | 3.45 ± 0.37 | 3.46 ± 0.43 | 0.83 |
| Final morphology | |||||||||
| Thrombus | 4 (2%) | 12 (6%) | 0.04 | 2 (2%) | 2 (2%) | >0.99 | 4 (4%) | 8 (8%) | 0.32 |
| Distal embolization | 7 (4%) | 22 (11%) | 0.004 | 1 (1%) | 6 (7%) | 0.06 | 10 (11%) | 12 (12%) | 0.87 |
| Slow flow or no reflow | 7 (4%) | 26 (14%) | 0.0006 | 4 (4%) | 3 (3%) | >0.99 | 8 (9%) | 18 (18%) | 0.07 |
| TIMI flow grade | |||||||||
| 0/1 | 6 (3%) | 10 (5%) | 0.32 | 1 (1%) | 5 (5%) | 0.11 | 3 (3%) | 7 (7%) | 0.34 |
| 2 | 11 (6%) | 27 (14%) | 0.007 | 6 (6%) | 5 (5%) | 0.81 | 8 (9%) | 19 (19%) | 0.05 |
| 3 | 173 (91%) | 156 (81%) | 0.004 | 90 (93%) | 83 (89%) | 0.39 | 80 (88%) | 76 (75%) | 0.02 |
| Myocardial blush grade | |||||||||
| 0/1 | 24 (13%) | 64 (34%) | <0.0001 | 13 (14%) | 11 (12%) | 0.77 | 29 (32%) | 35 (35%) | 0.65 |
| 2/3 | 161 (87%) | 127 (67%) | 0.45 | 82 (86%) | 79 (88%) | 0.77 | 62 (68%) | 65 (65%) | 0.65 |
| Corrected TIMI frame count (frames) | 17.9 ± 10.8 | 24.0 ± 18.0 | <0.0001 | 17.9 ± 10.9 | 17.9 ± 10.8 | 0.97 | 21.4 ± 12.9 | 26.3 ± 21.3 | 0.058 |
| Angiographic success | 173 (91%) | 155 (80%) | 0.003 | 90 (93%) | 83 (89%) | 0.39 | 80 (88%) | 75 (74%) | 0.01 |
| Procedural success | 188 (99%) | 190 (98%) | >0.99 | 96 (99%) | 92 (99%) | >0.99 | 90 (99%) | 100 (98%) | >0.99 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


