Electrical failure is more common in single-coil compared with dual-coil implantable cardioverter defibrillator (ICD) leads in the case of the recalled Riata lead. Single-coil leads are however favored in most patients given their lower risk of extraction. We therefore evaluated the failure-free survival of single- versus dual-coil ICD leads not included in Food and Drug Administration recalls. All patients receiving a Medtronic transvenous Sprint Quattro single- or dual-coil ICD lead were included in this analysis. Leads were followed to the end point of electrical failure. A total of 1,020 dual-coil and 631 single-coil ICD leads were implanted at our institution from November 2000 to March 2014. As expected, dual-coil leads had a longer follow-up time (3.4 ± 2.6 years vs 1.3 ± 1.0 years, p <0.001) because they were approved many years earlier by the Food and Drug Administration. The overall lead survival rates free from electrical failure at 1, 2, and 3 years after implantation were 98.8%, 98.2%, and 95.1%, respectively, for the single-coil leads versus 99.7%, 99.4%, and 99.3%, respectively, for the dual-coil leads (p = 0.0013). In conclusion, single-coil leads are associated with higher electrical failure rates compared with dual-coil leads even for nonrecalled lead models from the same family and manufacturer. These findings have implications on the choice of ICD lead at the time of device implantation.
Implantable cardioverter defibrillator (ICD) therapy is indicated for secondary and primary prevention of sudden cardiac death and has been shown to decrease all-cause mortality. The indications for implantation of these devices have expanded dramatically over the past 2 decades. Although defibrillator systems are very reliable, many ICD leads have exhibited failure rates that are greater than expected, resulting in Food and Drug Administration advisories and recalls. Lead extractions are relatively high-risk procedures that can be associated with major complications and death. Extraction of dual-coil versus single-coil ICD leads has been associated with significantly higher rates of major complications including mortality in return for minimal gain in terms of reduction in defibrillation thresholds. Based on these data, the use of single-coil leads is preferred in most patients. In the case of the Food and Drug Administration recalled Riata family of ICD leads, the survival of single-coil leads was significantly worse than that of dual-coil leads. The rate of electrical failure of single-coil Riata leads was 17%, compared with 4% to 7% for dual-coil leads from the same family. Whether this difference in lead survival between single- and dual-coil ICD leads is intrinsically related to the presence or absence of a proximal coil or whether it is specific to the Riata lead design is unclear. We therefore compare in this study the failure-free survival of single- versus dual-coil nonrecalled ICD leads from the same family and manufacturer and discuss the tradeoffs for using one versus the other at the time of ICD implantation.
Methods
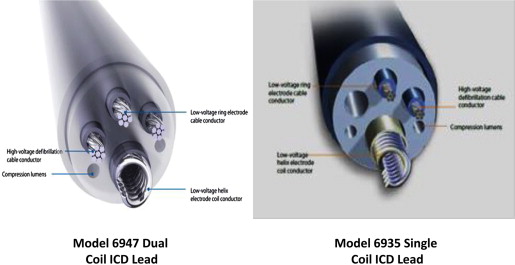
This study was approved by the Institutional Review Board at the University of Pittsburgh. Patients receiving a Medtronic transvenous Sprint Quattro (models 6947, 6935 and 6935M, Medtronic Inc., St. Paul, Minnesota) ICD lead at the University of Pittsburgh Medical Center from November 2000 to March 2014 were included. Patients implanted with the recalled Medtronic Fidelis lead (Medtronic model 6949, St. Paul, Minnesota) were excluded from this analysis. Both dual-coil (model 6947) and single-coil (models 6935 and/or 6935M) models are active fixation, dedicated bipolar ICD leads. They have similar primary silicon insulation and redundant insulation with ethylene tetrafluoroethylene for cables and polytetrafluoroethylene for coils. They are also similar in size with a lead diameter of 8.6 French and in right ventricular coil length and surface area. These lead models have an asymmetrical design with 4 cables in the dual-coil lead ( Figure 1 ) as opposed to 3 cables in the single-coil lead, which lacks a proximal coil. The dual-coil Sprint Quattro model 6947 was approved for use in the United States in 2001. The single-coil Sprint Quattro model 6935 was approved in 2008 and the 6935M model, which has a DF4 pin, was approved in 2012.
As per routine care at our institutions, patients were followed in the outpatient device clinic with no less than 1 clinic visit and 3 remote checks or 2 clinic visits annually for patients with no remote home monitoring. As previously described, leads were classified in follow-up as (1) functional lead, patient deceased (from any cause); (2) functional lead replaced for any reason other than electrical lead failure (e.g., infection, heart transplantation); (3) electrically failed lead, replaced; or (4) functional lead, active. Lead failure was defined as electrical malfunction resulting in lead extraction or replacement with a new ICD lead. Criteria for lead failure were similar to previously defined standards. Electrical malfunction was further classified as consisting of abnormal pacing, abnormal sensing, or abnormal high-voltage impedance values; increase in pacing threshold defined as intermittent or constant failure to capture or more than doubling of pacing threshold with a value >3.5 V at a pulse width of 0.5 ms with or without other associated electrical abnormalities, necessitating lead replacement; or the presence of electrical noise leading to inappropriate ICD therapy. Kaplan-Meier electrical failure-free survival curves were constructed for single- and dual-coil leads.
All continuous variables are presented as mean ± SD and are compared using the Student t test. All categorical variables are presented as absolute numbers or percentages and are compared using the chi-square test. Lead survival for single- and dual-coil ICD leads were evaluated using the Kaplan-Meier method and compared using the log-rank test. A 2-sided p value <0.05 was considered statistically significant. All statistical analyses were performed on SPSS, version 19.0.0 (IBM Inc., Armonk, New York).
Results
During the study period, 1,651 patients underwent placement of nonrecalled Medtronic ICD leads. Of those, 1,020 received dual-coil leads (Medtronic model 6947, St. Paul, Minnesota) whereas 631 received single-coil leads (Medtronic model 6935 and/or 6935M, St. Paul, Minnesota). Table 1 lists the baseline characteristics of patients receiving single- versus dual-coil leads. As expected, dual-coil leads had a longer follow-up time (3.4 ± 2.6 years vs 1.3 ± 1.0 years, p <0.001), because they were released on the United States market several years before the single-coil leads.
| Variables | Single-coil leads (n=631) | Dual-coil leads (n=1020) | P Value |
|---|---|---|---|
| Age at implantation (years) | 63 ± 15 | 64 ± 14 | 0.42 |
| Women | 26% | 26% | 0.65 |
| Ejection Fraction (%) | 33± 14 | 30 ± 13 | <0.001 |
| Coronary artery disease | 57% | 64% | 0.003 |
| Hypertension | 60% | 56% | 0.043 |
| Diabetes Mellitus | 32% | 34% | 0.54 |
| Secondary prevention defibrillator indication | 21% | 25% | 0.06 |
| Beta-blockers | 91% | 84% | <0.001 |
| Angiotensin converting enzyme inhibitors or angiotensin receptor blockers | 73% | 76% | 0.16 |
| Cardiac resynchronization Therapy device | 35% | 38% | 0.24 |
| Follow-up (years) | 1.3 ± 1.0 | 3.4 ± 2.6 | <0.001 |
The overall lead survival rates free from electrical failure at 1, 2, and 3 years after implantation were 98.8%, 98.2%, and 95.1%, respectively, for the single-coil leads versus 99.7%, 99.4%, and 99.3%, respectively, for the dual-coil leads (p = 0.0013; Figure 2 ). As listed in Table 2 , there were 12 electrical failures in single-coil leads, including high pacing thresholds (n = 4), significant change in pacing impedance (n = 4), ventricular oversensing (n = 2), and high-voltage failures (n = 2). For the dual-coil leads, there were 11 lead failures, including significant change in pacing impedance (n = 8) and ventricular oversensing (n = 3).




