The aim of this study was to assess the effect of testosterone replacement therapy (TRT) on cardiovascular outcomes. Men (January 1, 1996, to December 31, 2011) with a low initial total testosterone concentration, a subsequent testosterone level, and >3 years of follow-up were studied. Levels were correlated with testosterone supplement use. The primary outcome was major adverse cardiovascular events (MACE), defined as a composite of death, nonfatal myocardial infarction, and stroke at 3 years. Multivariate adjusted hazard ratios (HRs) comparing groups of persistent low (<212 ng/dl, n = 801), normal (212 to 742 ng/dl, n = 2,241), and high (>742 ng/dl, n = 1,694) achieved testosterone were calculated by Cox hazard regression. A total of 4,736 men were studied. Three-year rates of MACE and death were 6.6% and 4.3%, respectively. Subjects supplemented to normal testosterone had reduced 3-year MACE (HR 0.74; 95% confidence interval [CI] 0.56 to 0.98, p = 0.04) compared to persistently low testosterone, driven primarily by death (HR 0.65, 95% CI 0.47 to 0.90). HRs for MI and stroke were 0.73 (95% CI 0.40 to 1.34), p = 0.32, and 1.11 (95% CI 0.54 to 2.28), p = 0.78, respectively. MACE was noninferior but not superior for high achieved testosterone with no benefit on MI and a trend to greater stroke risk. In conclusion, in a large general health care population, TRT to normal levels was associated with reduced MACE and death over 3 years but a stroke signal with high achieved levels suggests a conservative approach to TRT.
Testosterone has been approved in the United States since the 1950s as replacement therapy for male hypogonadism. Beyond relatively uncommon cases of classical hypogonadism, many otherwise healthy men experience a decrease in serum concentrations of testosterone with age. Although the decrease is usually modest, levels may fall below the lower limit of the normal range for younger, healthy men, a condition referred to as “andropause” or “age-related hypogonadism.” Furthermore, aging men often experience signs and symptoms associated with hypogonadism, including reduced levels of energy, sexual function, muscle mass and strength, and bone mineral density and increases in fat mass. Epidemiologic studies have identified low testosterone in middle-aged and older men as an independent risk factor for cardiovascular (CV) disease, cancer, and total mortality. In response to an aging population, a desire to maintain youthful health and energy, and direct-to-consumer advertising, the use of testosterone replacement therapy (TRT) has increased dramatically in recent years. The US Food and Drug Administration (FDA) has estimated that testosterone supplements sold from 2009 to 2013 increased 65%, and the number of patients receiving TRT prescriptions increased from 1.3 million to 2.3 million, with men 40- to 60-year old accounting for 70% of prescriptions. Despite this trend, the extent to which age-related symptoms are a consequence of low testosterone levels and for which TRT is clinically effective is unclear. Furthermore, the impact of TRT on CV outcomes is controversial and largely based on limited, mostly retrospective study data with disparate conclusions. The incomplete and conflicting data from these studies have led to contrasting international regulatory agency conclusions as to the risk versus benefit of TRT. Given this lack of clarity in its CV risk impact, we sought to assess the safety of TRT prescribed by licensed physicians for symptomatic patients across a large, integrated health care system.
Methods
The study prespecified the following primary and secondary hypotheses:
- 1.
Men with documented testosterone deficiency replaced to the normal range with TRT have no greater risk of death, myocardial infarction (MI), or stroke (major adverse CV event [MACE]) at 1 and 3 years (primary outcome) than those who remain at low testosterone.
- 2.
The risk of MACE will be increased for those with (1) persistently low testosterone and (2) an excessively high testosterone compared to those in whom testosterone is normalized.
- 3.
The relative risk of CV outcomes of testosterone-deficient men replaced to normal testosterone is unaffected by age (<65 vs ≥65 years).
- 4.
In men with preexisting coronary artery disease (CAD), MI, or stroke and low testosterone, TRT to normal testosterone is not associated with an increased risk of MACE.
Men who received care at an Intermountain Healthcare facility, were aged ≥50 years of age, had a documented low testosterone level (<212 ng/dl) and a follow-up testosterone, and at least 3 years of follow-up were identified. Those with a baseline diagnosis of malignancy (excluding basal and squamous cell carcinoma) were excluded because of potential confounding interactions with testosterone levels and therapies, resulting in a study cohort of 4,736 subjects. For primary and secondary hypothesis testing: (1) All qualifying men within Intermountain Healthcare with a first documented low testosterone level (<212 ng/dl; with time of test designated as the study entry date) and at least 3 years of electronic medical records follow-up were identified. (2) These men were then verified as receiving (or not) TRT by documentation of a testosterone prescription. (3) Men were categorized by achieved testosterone level (ng/ml) into 3 categories: <212 (low), 212 to 742 (normal), >742 (high). Patients were then followed for study end points at 1 and 3 years, starting from the date of the documentation of initiation of TRT or baseline testosterone in others who did not receive TRT.
Intermountain Healthcare is a large, electronically integrated health care organization that provides most of the health care for the state of Utah and southeastern Idaho. Intermountain’s large electronic medical record database warehouse, comprising over 3 million patient records, including laboratory tests, medications, and patient diagnoses and outcomes, was searched from 1996 to 2011 to identify men meeting study inclusion and exclusion criteria. The study was approved by the Intermountain Urban Central Institutional Review Board.
Blood analyte testing was performed by the CLIA-certified (federal regulatory standards that apply to all clinical laboratory testing) Intermountain Healthcare Central Laboratories using standard methods. Serum total testosterone concentration was categorized as low (<212 ng/dl), normal (212 to 742 ng/dl), and high (>742 ng/dl) according to laboratory and manufacturer specifications. Testosterone measurement used a chemiluminescent competitive immune assay. Briefly, a serum sample is mixed with labeled testosterone and a solid-phase antitestosterone antibody. After the mixture achieves equilibrium, bound and free testosterone fractions are separated, and the amount of bound label is measured by the generation of a luminescent signal. Quantitation of testosterone in the sample then is determined by comparison to a standard curve. Because different laboratories may have differing ranges of normal, the laboratory’s specified ranges rather than numerical stratification should be used to make comparisons between studies.
The primary independent subgrouping variable was the achieved testosterone level on follow-up, defined as persistently low, normal, or high. The primary end point of the study was the composite of all-cause death or nonfatal MI or stroke (MACE). Secondary end points were the individual components of MACE, type of death (CV, coronary, and cancer), and the composite of CV death, nonfatal MI, or stroke (CV-MACE). Death was determined by electronic medical records review, Utah Health Department death certificate records, and the national Social Security Death Index. Cause of death was only available in those who had a Utah State death certificate. CV death was defined as death from MI, stroke, heart failure, presumed arrhythmia (sudden death), or death not known to be due to a non-CV cause. Coronary death included fatal MI, sudden ischemic death, and any other death presumed to be related to coronary atherosclerosis. Nonfatal MI was defined as a hospitalization associated with a troponin I level >0.4 ng/ml or a discharge diagnosis of MI ( International Classification of Diseases [ICD] 9 code 410; ICD-10 codes: I21, I22, I23). Stroke also was determined by ICD codes (ICD-9 code 433.1 and 434.1; ICD-10 codes: I60 to I64).
The Student t test, analysis of variance, and chi-square statistic were used as appropriate to compare baseline characteristics defined by achieved testosterone levels. The chi-square statistic and Fisher’s exact test were also used to compare 1- and 3-year outcomes in the groups. Multivariate Cox hazard regression tests were used to determine the adjusted risk of the outcomes by achieved testosterone levels. Variables used in the modeling included age, hypertension, hyperlipidemia, smoking, diabetes, renal failure, previous CAD, previous MI, previous stroke, atrial fibrillation, heart failure, peripheral vascular disease, previous pulmonary embolism, chronic obstructive pulmonary disease, baseline testosterone level, angiotensin-converting enzyme inhibitors, angiotensin renin blockers, calcium channel blockers, diuretics, and statins. Subanalyses were performed in those who had a Charlson Comorbidity Index (CCI) score, with the CCI used a model covariable. Only significant and confounding variables (i.e., associated with at least a 10% change in the β coefficient) were included in the final models. The noninferiority 95% confidence interval (CI) margin of the 3-year hazard ratio (HR) was designated as a delta of 0.2. When noninferiority was achieved, testing for superiority was performed.
Results
A total of 4,736 qualifying men with low baseline testosterone were identified and studied. Baseline characteristics are summarized in Table 1 . Overall age averaged 61.2 ± 8.7 years, 27.6% were diabetics, and 20.7% had documented CAD. On follow-up testosterone testing (at a median of 742 days after baseline), study patients were categorized as follows: persistently low (<212 ng/dl, n = 801), normal (212 to 742 ng/dl, n = 2,241), and high achieved testosterone levels (>742 ng/dl, n = 1,694). TRT was provided to only 18% of those who with persistently low testosterone and to all with normal or high achieved testosterone. Of the different modes of testosterone therapy, gel was the primary mode used for supplementation (gel: 90%, injection: 9.0%, oral pill: 1.0%).
| Characteristic | Testosterone Levels (ng/dL) | p-value | ||
|---|---|---|---|---|
| <212 (n=801) | 212-742 (n=2,241) | >742 (n=1,694) | ||
| Age (years) | 62.8±9.7 | 61.2±8.3 | 60.4±8.5 | <0.0001 |
| Hypertension | 58.3% | 57.0% | 53.2% | 0.02 |
| Hyperlipidemia | 54.7% | 59.5% | 58.6% | 0.06 |
| Diabetes mellitus | 32.6% | 29.3% | 23.0% | <0.0001 |
| Smoke | 20.3% | 18.2% | 16.5% | 0.06 |
| History of coronary artery disease (stenosis ≥70%) | 25.0% | 20.0% | 19.7% | 0.005 |
| Prior myocardial infarction | 5.7% | 3.9% | 3.5% | 0.03 |
| History of heart failure | 12.1% | 8.2% | 6.8% | <0.0001 |
| Prior stroke | 2.4% | 1.6% | 1.8% | 0.33 |
| Peripheral artery disease | 2.7% | 2.5% | 2.0% | 0.47 |
| Atrial fibrillation | 7.9% | 7.4% | 7.9% | 0.85 |
| Prior pulmonary embolism | 2.6% | 2.7% | 2.4% | 0.77 |
| Chronic obstructive pulmonary disease | 10.1% | 4.9% | 3.2% | <0.0001 |
| Renal failure | 6.3% | 4.8% | 3.7% | 0.02 |
| Body metabolic index (kg/m 2 ), n=1,192 | 33.1±7.1 | 32.4±7.8 | 32.0±6.9 | 0.27 |
| Angiotensin Converting Enzyme Inhibitor ∗ | 12.0% | 12.4% | 10.0% | 0.07 |
| Angiotensin II Receptor Blocker ∗ | 5.5% | 5.0% | 5.8% | 0.59 |
| Calcium Channel Blocker ∗ | 6.2% | 6.2% | 5.7% | 0.75 |
| Diuretic ∗ | 16.0% | 11.9% | 11.3% | 0.003 |
| Statin ∗ | 14.7% | 16.1% | 16.1% | 0.63 |
| Baseline testosterone | 121.7±66.1 (median: 139) | 144.3±60.8 (median: 164) | 141.8±56.8 (median: 158) | <0.0001 |
| Intermountain Mortality Risk Score, n=1,100 | 10.0±4.0 | 9.5±3.9 | 9.9±3.6 | 0.21 |
| Charlson Comorbidity Index, n=3,071 | 2.8±2.1 | 2.5±2.0 | 2.4±1.7 | <0.0001 |
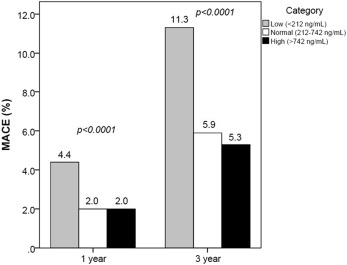
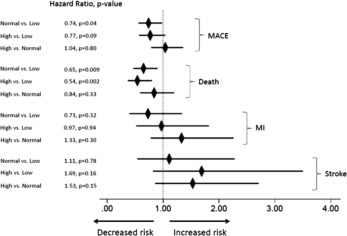
MACE event rates at 1 and 3 years by testosterone group are summarized in Table 2 and Figure 1 . Overall 3-year rates of MACE and death were 6.6% and 4.3%, respectively. Multivariate adjusted HRs by testosterone group are presented in Figure 2 and Table 3 . Noninferiority was achieved for the primary hypothesis test comparing normal attained testosterone to persistently low testosterone. Noninferiority also was achieved individually for all-cause death but not MI or stroke when comparing normal to low. Subsequent superiority testing indicated better outcomes for 3-year MACE (HR 0.74, 95% CI 0.56 to 0.98, p = 0.04), which was driven primarily by superiority for all-cause death (HR 0.65, 95% CI 0.47 to 0.90, p = 0.009).
| Testosterone Levels (ng/dL) | |||
|---|---|---|---|
| <212 (n=801), 18% supplemented | 212-742 (n=2,241), 100% supplemented | >742 (n=1,694), 100% supplemented | |
| Major adverse cardiovascular events | |||
| 1 year b | 4.4% (35) | 2.0% (44) | 2.0% (34) |
| 3 years b | 11.3% (91) | 5.9% (132) | 5.3% (90) |
| All-cause death | |||
| 1 year b | 2.9% (23) | 1.3% (29) | 0.7% (11) |
| 3 years b | 8.9% (71) | 3.8% (86) | 2.8% (47) |
| Myocardial infarction | |||
| 1 year a | 1.4% (11) | 0.3% (7) | 0.6% (10) |
| 3 years | 2.2% (18) | 1.3% (30) | 1.5% (26) |
| Stroke | |||
| 1 year | 0.6% (5) | 0.5% (11) | 0.9% (15) |
| 3 years | 1.5% (12) | 1.1% (25) | 1.5% (25) |
| Coronary death, n=4,650 | |||
| 1 year | 0.8% (6/774) | 0.4% (8/2212) | 0.2% (4/1,664) |
| 3 years a | 2.2% (17/774) | 0.7% (16/2,212) | 0.9% (15/1,664) |
| Cardiovascular death, n=4,650 | |||
| 1 year | 1.2% (9/774) | 0.6% (14/2,212) | 0.4% (7/1,664) |
| 3 years a | 3.5% (27/774) | 1.6% (35/2,212) | 1.6% (26/1,664) |
| Cardiovascular major adverse cardiovascular events, n=4,650 | |||
| 1 year a | 2.7% (21/774) | 1.3% (28/2,212) | 1.8% (30/1,664) |
| 3 years a | 6.1% (47/774) | 3.7% (81/2,212) | 4.1% (69/1,664) |
| Cancer death, n=4,650 | |||
| 1 year | 0.1% (1/774) | 0.2% (4/2,212) | 0% (0/1,664) |
| 3 years a | 1.8% (14/774) | 0.9% (21/2,212) | 0.4% (7/1,664) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


