Transarterial aortic valve implantation (TAVI) is a promising method for the treatment of high-risk patients with aortic stenosis. Because gender differences are known in aortic stenosis, the aim of this study was to compare procedural and short-term outcomes, left ventricular remodeling, and inflammatory status after TAVI in men and women. One hundred consecutive patients (42 men, 58 women) who underwent transfemoral TAVI (CoreValve in 83%, SAPIEN in 17%) were prospectively analyzed. Aortic stenosis severity was higher in women (mean valve area 0.7 ± 0.3 vs 0.8 ± 0.2 cm 2 ). Women had better ejection fractions, smaller end-diastolic and end-systolic diameters, and more concentric hypertrophy at baseline. There were no differences in device success rate (99%), 30-day total mortality (2.4% in men, 3.4% in women), stroke (2.4% in men, 1.7% in women), or pacemaker rate (26.2% in men, 15.5% in women). Periprocedural complications and 3-month outcome were not different between the genders. After TAVI, regression of hypertrophy occurred in men and women, but improvement of the ejection fraction was significant only in women. N-terminal pro–B-type natriuretic peptide decreased to similar levels in the 2 genders. C-reactive protein and interleukin-6, elevated at baseline more in men than in women, decreased after TAVI and normalized at 3 months only in women. In conclusion, women clinically benefit from TAVI to a degree similar to that of men. However, there are gender differences involving the recovery response of the left ventricle after TAVI.
Only limited data are available concerning gender differences with regard to transarterial aortic valve implantation (TAVI). Data from the Milan registry suggested that there are no gender differences in all-cause and cardiovascular mortality. Recently, Hayashida et al demonstrated that female gender is associated with better midterm survival after TAVI. The purpose of our study was to obtain further insight into outcomes in women and men, and because little is known regarding gender-specific differences in left ventricular (LV) response to acute mechanical unloading, we especially focused on echocardiographic parameters of LV geometry and function as well as on neurohumoral and inflammatory mediators possibly involved in myocardial remodeling processes after transfemoral TAVI.
Methods
From July 2009 to July 2011, procedural and short-term outcomes of consecutive female and male patients after transfemoral TAVI were compared at Charité University Hospital, Campus Mitte (Berlin, Germany). Patients were treated with TAVI if the aortic valve area was <1 cm 2 , the European System for Cardiac Operative Risk Evaluation score (EuroSCORE) was >20%, or the European System for Cardiac Operative Risk Evaluation was <20% if ≥1 of the following criteria was met: contraindication to surgery, severely reduced pulmonary function, liver cirrhosis, or metastatic cancer. CoreValve (Medtronic Inc., Minneapolis, Minnesota) and SAPIEN (Edwards Lifesciences, Irvine, California) prostheses were used. The techniques for TAVI have been described previously. The Prostar XL vessel-closure system (Abbott Vascular, Abbott Park, Illinois) was used.
Cardiovascular history, functional evaluation, and echocardiographic and invasive data were assessed before TAVI using standard techniques. Echocardiographic measurements were performed according to the American Society of Echocardiography and European Society of Echocardiography guidelines. LV mass (LVM) was calculated according to the Devereux formula. LVM was indexed to body surface area. LVM index exceeding 110 g/m 2 in women and 125 g/m 2 in men was defined as LV hypertrophy. Calculation of relative wall thickness (RWT) was assessed by the formula (2 × diastolic posterior wall thickness)/LV end-diastolic diameter. RWT was used to categorize LV hypertrophy as either concentric (RWT ≥0.42) or eccentric (RWT <0.42). RWT ≥0.42 without an increase in LVM index defined concentric remodeling. Procedural, 30-day, and 3-month end points were analyzed according to the recently proposed Valve Academic Research Consortium end point definitions. Echocardiographic parameters, N-terminal pro–B-type natriuretic peptide (NT-proBNP) (Elecsys; Roche Diagnostics GmbH, Mannheim, Germany), interleukin-6 (IL-6), tumor necrosis factor–α (both IMMULITE; Siemens, Eschborn, Germany), and C-reactive protein (CRP) (Roche Diagnostics GmbH) were assessed at baseline, day 7, and 3 months after TAVI. NT-proBNP values from patients with dialysis and with creatinine >1.5 mg/dl were excluded.
Results are expressed as arithmetic mean ± SD, median (interquartile range), or frequency (percentage). After checking the distributions for normality, differences between the groups studied were investigated with respect to interesting clinical parameters by using nonparametric exact Mann-Whitney tests for independent groups or exact Wilcoxon’s tests for pairwise comparisons. Frequencies were determined using exact Mantel-Haenszel tests (ordered categories) or exact chi-square tests in contingency tables. Differences in complications were determined using univariate and multivariate logistic regression. Changes in clinical outcomes with respect to time were analyzed using multivariate nonparametric analysis of longitudinal data with a 2-factorial design (first [independent] factor: gender; second [dependent] factor: repetitions in time) (multivariate analysis of variance). All time points were simultaneously compared. Moreover, we performed calculations with adjustments for baseline influences using a nonparametric multivariate analysis of covariance with the baseline as covariate. Post hoc analyses were carried out to detect specific differences with respect to gender for fixed times (Mann-Whitney tests). A 2-tailed p value <0.05 was considered statistically significant.
Results
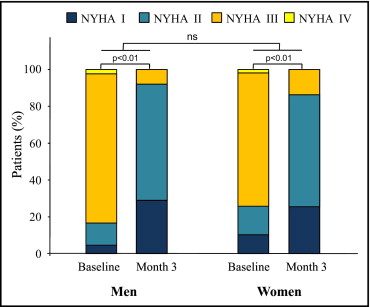
Baseline characteristics are listed in Table 1 . Women were slightly older (p = NS) and were smaller in height (162 ± 7 vs 173 ± 7 cm), weight (67 ± 14 vs 82 ± 17 kg), and body surface area than men. With regard to co-morbidities, chronic renal insufficiency was present in 74% of men and 60% of women; 7% of men and 3% of women were on chronic dialysis. Coronary artery disease and diabetes were more frequent in men, whereas chronic obstructive pulmonary disease was more common in women. Distribution of New York Heart Association classes was comparable, with most patients in class III ( Figure 1 ).
| Variable | All | Men | Women | p Value ⁎ |
|---|---|---|---|---|
| (n = 100) | (n = 42) | (n = 58) | ||
| Age (years) | 79 ± 8 | 77 ± 9 | 80 ± 8 | NS |
| Body surface area (m 2 ) | 1.8 ± 0.2 | 2.0 ± 0.2 | 1.7 ± 0.2 | <0.01 |
| Body mass index (kg/m 2 ) | 26 ± 5 | 27 ± 5 | 26 ± 6 | NS |
| Logistic EuroSCORE (%) | 19.9 ± 15.4 | 22.1 ± 18.3 | 18.4 ± 12.8 | NS |
| Pulmonary hypertension | 56% | 57.1% | 55.2% | NS |
| Systolic pulmonary arterial pressure (mm Hg) | 46 ± 17 | 49 ± 18 | 45 ± 16 | NS |
| Glomerular filtration rate (ml/min) | 60.5 ± 24.7 | 56.5 ± 21.1 | 63.3 ± 26.8 | NS |
| Coronary artery disease | 58% | 79% | 43% | <0.01 |
| Previous coronary artery bypass graft | 12% | 21% | 5% | <0.05 |
| Previous myocardial infarction | 15% | 26% | 7% | <0.01 |
| Previous percutaneous coronary intervention | 41% | 62% | 26% | <0.01 |
| Porcelain aorta | 8% | 5% | 10% | NS |
| Peripheral artery disease | 29% | 33% | 26% | NS |
| Previous transitory ischemic attack | 3% | 2% | 3% | NS |
| Previous stroke | 10% | 12% | 9% | NS |
| Arterial hypertension | 91% | 93% | 90% | NS |
| Diabetes mellitus | 45% | 57% | 36% | <0.05 |
| Hyperlipidemia | 61% | 67% | 57% | NS |
| Atrial fibrillation | 19% | 14% | 22% | NS |
| Chronic obstructive pulmonary disease | 34% | 21% | 43% | <0.05 |
| Pacemaker before TAVI | 17% | 17% | 17% | NS |
| Automatic implantable cardioverter-defibrillator before TAVI | 3% | 7% | 0% | NS |
Echocardiographic analysis revealed narrower aortic annulus diameters and smaller aortic valve areas in women than in men. Ejection fractions (EFs) were significantly higher in women. With regard to invasive data, cardiac output was lower in women, but this difference disappeared after adjustment for body surface area. As expected, the diameter of the common femoral artery was smaller in women ( Table 2 ).
| Variable | All | Men | Women | p Value ⁎ |
|---|---|---|---|---|
| Aortic valve area (cm 2 ) | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.7 ± 0.3 | <0.01 |
| Aortic valve area index (cm 2 /m 2 ) | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.2 | NS |
| Annulus diameter (cm 2 ) | 23.5 ± 2.6 | 24.6 ± 2.2 | 22.7 ± 2.6 | <0.01 |
| Mean aortic gradient (mm Hg) | 44.4 ± 18.4 | 43.0 ± 15.9 | 45.4 ± 20.0 | NS |
| Peak aortic gradient (mm Hg) | 74.3 ± 28.2 | 72.4 ± 25.3 | 75.6 ± 30.2 | NS |
| Vmax (m/s) | 4.3 ± 0.8 | 4.2 ± 0.8 | 4.3 ± 0.9 | NS |
| Aortic regurgitation | ||||
| 0 | 24% | 22% | 25% | NS |
| 1 | 63% | 61% | 65% | NS |
| 2 | 13% | 17% | 10% | |
| 3 | 0 | 0 | 0 | |
| EF (%) | 51.1 ± 12. 0 | 46.7 ± 14.8 | 54.3 ± 8.4 | <0.01 |
| EF ≤40% | 19% | 36% | 7% | <0.01 |
| Cardiac output (l/min) | 3.8 ± 1.0 | 4.2 ± 0.9 | 3.5 ± 0.9 | <0.01 |
| Cardiac index (l/min/m 2 ) | 2.1 ± 0.5 | 2.1 ± 0.5 | 2.0 ± 0.5 | NS |
| Pulmonary capillary wedge pressure (mm Hg) | 19.1 ± 8.6 | 21.1 ± 10.6 | 17.6 ± 7.2 | NS |
| Common femoral artery diameter (mm) | 6.9 ± 1.0 | 7.6 ± 0.9 | 6.5 ± 0.8 | <0.01 |
Procedural details are listed in Table 3 . A total of 83% of the patients received CoreValve devices, and 17% received SAPIEN prostheses. In accordance with their narrower aortic dimensions, significantly smaller valves were implanted in women. Device success was achieved in 99% of patients. The only device failure and procedural death occurred in an 86-year-old woman presenting with severe systemic sclerosis and pulmonary hypertension (EuroSCORE 30%). In this patient, left main occlusion occurred during CoreValve deployment. The valve was immediately retrieved into the ascending aorta using a snare device, and the procedure was switched to open aortic valve replacement. Despite assist device support, the patient died 48 hours later. There were no cases of valve embolization. However, 2 men underwent delayed conventional aortic valve replacement, 1 because of late valve migration (SAPIEN, 26 mm) and 1 because of progressive paravalvular regurgitation (CoreValve, 29 mm), both 2 months after TAVI ( Table 3 ). There were 3 episodes of tamponade, which occurred exclusively in the first 10 patients while using a conventional lead for rapid pacing. After switching to a balloon-tip lead, no further episodes of tamponade were observed. There was a trend toward more bleeding in women. Life-threatening bleeding was 2.5-fold more frequent than in men, but this finding was not significant. The 30-day total mortality rate was low, at 3%, with no difference between genders. The 30-day pacemaker or implantable cardioverter-defibrillator rate for the entire group was 20%. Surprisingly, the pacemaker rates between CoreValve (21.5%) and SAPIEN (18.8%) prostheses were similar.
| Variable | All | Men | Women | p Value ⁎ |
|---|---|---|---|---|
| CoreValve | <0.01 | |||
| 26 mm | 37% | 10% | 57% | |
| 29 mm | 45% | 69% | 28% | |
| 31 mm | 1% | 0% | 2% | |
| SAPIEN | <0.01 | |||
| 23 mm | 6% | 0% | 10% | |
| 26 mm | 11% | 21% | 3% | |
| No device success | 1% | 0% | 1.7% | NS |
| Death (<48 hours) | 1% | 0% | 1% | NS |
| Death (<30 days) | 3% | 2.4% | 3.4% | NS |
| Conversion to surgery | NS | |||
| Acute | 1.0% | 0% | 1.7% | |
| Delayed | 2.0% | 4.8% | 0% | |
| Stroke | NS | |||
| Transient ischemic attack | 0% | 0% | 0% | |
| Major | 2% | 2.4% | 1.7% | |
| Minor | 0% | 0% | 0% | |
| Bleeding events | 0.07 | |||
| None or minor | 78.0% | 83.3% | 74.1% | |
| Major | 13.0% | 11.9% | 13.8% | |
| Life threatening | 9.0% | 4.8% | 12.1% | |
| Vascular access site complications | NS | |||
| None or minor | 92.0% | 92.9% | 91.4% | |
| Major | 8.0% | 7.1% | 8.6% | |
| Tamponade | 3% | 2.4% | 3.4% | NS |
| Periprocedural pacemaker (<48 hours) | 7% | 7.1% | 6.9% | |
| Pacemaker (<30 days) | 20% | 26.2% | 15.5% | NS |
Potential risk factors were investigated using multivariate logistic regression models. Narrow annulus diameter (p = 0.01), low glomerular filtration rate (p = 0.048), low EF (p = 0.018), and CRP (p = 0.032) emerged as independent predictors of procedural complications. Gender, however, was not a significant risk factor for acute or short-term complications.
As listed in Table 4 , there were no significant gender-specific differences in short-term outcomes up to 3 months. Hemodynamic results at 3 months were good, with no differences between the genders. No or minor residual aortic regurgitation was observed in 86%, without relevant gender-specific differences.
| Variables | All | Men | Women | p Value ⁎ |
|---|---|---|---|---|
| Total mortality | 8% | 7% | 9% | NS |
| Cardiovascular mortality | 5% | 7% | 3% | NS |
| Pacemaker † | 20% | 26% | 16% | NS |
| Transitory ischemic attack, minor stroke | 0% | 0% | 0% | |
| Major stroke | 2% | 2% | 2% | NS |
| Mean aortic gradient (mm Hg) | 9.0 ± 4.0 | 8.8 ± 3.6 | 9.2 ± 4.2 | NS |
| Aortic valve area (cm 2 ) | 1.7 ± 0.4 | 1.8 ± 0.4 | 1.6 ± 0.3 | <0.01 |
| Aortic valve area index (cm 2 /m 2 ) | 0.9 ± 0.2 | 1.0 ± 0.3 | 0.9 ± 0.2 | NS |
| Residual aortic regurgitation | NS | |||
| 0 | 23% | 21% | 25% | |
| 1 | 63% | 56% | 67% | |
| 2 | 14% | 23% | 8% | |
| 3 | 0% | 0% | 0% |
⁎ Differences between men and women.
† Including 2 automatic implantable cardioverter-defibrillators.
Table 5 lists changes in ventricular function and geometry. In the 2 genders, there were increases in EFs at 3-month follow-up. This amelioration was significant only for the female cohort. E/E′ ratio as indicator of diastolic function showed a trend toward higher values in women at each time point during follow-up. Eighty-three percent of men and women had increased LVM indexes at baseline. RWT was significantly higher in women, which indicates that women present more commonly with concentric hypertrophy ( Figure 2 ). Septal and posterior wall thickness, LVM, and LVM index had decreased significantly by 3-month follow-up, without relevant differences between the genders ( Table 5 ).
| Variable | Gender | Baseline | 7 Days | 3 Months | p Value ⁎ |
|---|---|---|---|---|---|
| EF (%) | Men | 46.7 ± 14.8 | 48.1 ± 14.0 | 49.6 ± 13.8 | NS |
| Women | 54.3 ± 8.4 | 56.4 ± 7.3 | 56.6 ± 6.6 | <0.01 | |
| p value † | <0.01 ‡ | <0.01 ‡ | 0.02 ‡ | ||
| E/E′ ratio | Men | 15.9 ± 5.4 | 18.4 ± 7.6 | 14.9 ± 5.6 | NS |
| Women | 19.0 ± 7.5 | 19.4 ± 6.9 | 17.7 ± 7.1 | NS | |
| p value † | 0.08 | NS | 0.05 | ||
| Posterior wall thickness (mm) | Men | 13.4 ± 1.7 | 12.8 ± 1.4 | 12.3 ± 1.8 | <0.01 |
| Women | 13.5 ± 2.2 | 13.2 ± 1.7 | 12.1 ± 1.7 | <0.01 | |
| p value † | NS | NS | NS | ||
| Interventricular septal thickness (mm) | Men | 13.9 ± 2.0 | 13.1 ± 2.0 | 12.4 ± 1.8 | <0.01 |
| Women | 14.3 ± 2.2 | 13.6 ± 2.1 | 13.1 ± 1.7 | <0.01 | |
| p value † | NS | NS | NS | ||
| RWT | Men | 0.53 ± 0.12 | 0.51 ± 0.10 | 0.48 ± 0.10 | 0.053 |
| Women | 0.65 ± 0.18 | 0.59 ± 0.14 | 0.54 ± 0.12 | <0.01 | |
| p value † | <0.01 | <0.01 | 0.058 | ||
| End-diastolic diameter (mm) § | Men | 51.1 ± 7.8 | 50.6 ± 7.6 | 51.2 ± 7.4 | NS |
| Women | 42.9 ± 7.2 | 45.1 ± 6.7 | 45.2 ± 6.7 | 0.20 | |
| End-systolic diameter (mm) § | Men | 36.5 ± 10.7 | 33.9 ± 9.7 | 34.7 ± 8.8 | <0.01 |
| Women | 26.9 ± 8.8 | 28.7 ± 7.1 | 28.3 ± 6.7 | <0.01 | |
| LVM (g) § | Men | 294 ± 74 | 266 ± 57 | 265 ± 63 | 0.03 |
| Women | 231 ± 61 | 236 ± 59 | 216 ± 57 | 0.01 | |
| LVM index (g/m 2 ) § | Men | 149 ± 31 | 136 ± 28 | 135 ± 26 | 0.02 |
| Women | 135 ± 34 | 135 ± 29 | 125 ± 30 | 0.02 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


