Acute kidney injury (AKI) is relatively common after cardiothoracic surgery for type A acute aortic dissection (TA-AAD) and increases mortality. We investigated the incidence and risk factors for AKI in patients with TA-AAD and its impact on their outcomes. The records of 375 consecutive patients who underwent surgical treatment for TA-AAD from October 2007 to March 2013 were analyzed retrospectively. We defined AKI using the Kidney Disease Improving Global Outcomes criteria, which are based on serum creatinine concentration or glomerular filtration rate. We used Kaplan-Meier methods and multivariate Cox proportional hazards regression to assess the impact of AKI on both mortality and major adverse cardiovascular and cerebrovascular events. We also examined the association between risk factors and AKI using logistic regression modeling. Postoperative AKI was observed in 165 patients (44.0%). The overall 30-day and mid- to long-term mortality was 1.6% and 8.8%, respectively. Mortality and major adverse cardiovascular and cerebrovascular events correlated significantly with the severity of AKI, and multivariate analysis showed that AKI stage 3 (the most sever stage) was an independent risk factor for mortality (hazard ratio 6.83, 95% confidence interval 2.52 to 18.52) after adjustment for important confounding factors. Extracorporeal circulation time, body mass index, perioperative peak serum C-reactive protein concentration, renal malperfusion, and perioperative sepsis were found to be risk factors for AKI. In conclusion, AKI was common in patients who underwent surgery for type A acute aortic dissection. The severity of AKI strongly influences patient outcomes, so it should be recognized promptly and treated aggressively when possible.
Despite recent advances in medical management and surgical techniques, type A acute aortic dissection (TA-AAD) still carries a high burden of morbidity and mortality. In addition to many predictors previously reported, acute kidney injury (AKI) has received recent attention as an important risk factor for mortality after TA-AAD ; however, the diagnostic criteria and classification systems used to define AKI in previous studies have not been consistent. Furthermore, patient populations have been relatively small and the length of follow-up relatively short, raising the possibility that previous studies lacked sufficient statistical power. The primary aims of this study were to clarify the incidence of AKI in a large population of patients who received surgical treatment for TA-AAD and to investigate its impact on both short- and mid- to long-term outcomes.
Methods
We reviewed the medical records and the results of laboratory investigations of 418 consecutive patients admitted to the Sakakibara Heart Institute (a 320-bed cardiac center in Tokyo, Japan) diagnosed with TA-AAD according to the Stanford classification from October 2007 to March 2013. Previous approval was obtained from the institutional research ethics committee (approval number: 13-31), which waived the need for individual informed consent. Aortic dissection was diagnosed by computed tomography angiography at either our institution or the referring hospital. None of the patients was in cardiopulmonary arrest at the time of admission; 16 patients who did not undergo surgery were excluded from the analysis: in 5, surgery was considered to be contraindicated because of advanced age or a history of end-stage cancer and 11 did not meet the criteria for surgery. We selected these patients for medical management according to the guidelines of the Japanese Circulation Society, which identifies those with noncommunicating TA-AAD with an ascending aortic diameter <45 mm and without aortic regurgitation or cardiac tamponade as being suitable for a conservative treatment strategy. We also excluded 4 patients who had already received renal replacement therapy at the time of admission. The final study population consisted of 375 patients.
AKI was diagnosed using the Kidney Disease Improving Global Outcomes (KDIGO) criteria with minor modifications ( Table 1 ). According to the KDIGO criteria, perioperative AKI is defined by severity on the basis of the maximal change in the serum creatinine (sCr) concentration during the first postoperative week compared with preoperative baseline values. Therefore, 23 patients who died within the first to seventh postoperative days were also excluded from our analysis. (The majority of those patients died within 2 days after operation because of surgical mechanical complications that have no relevance to AKI. Therefore, removal of those patients will not affect our analysis.) The original KDIGO criteria also use urine output to define AKI. We chose not to use urine output to define AKI in our study. In our opinion, urine output data are not reliable when collected retrospectively, and recent reports have suggested that sCr is a superior predictor of AKI and subsequent outcomes.
| Stage | serum Creatinine (sCr) Increase |
|---|---|
| 1 | 1.5 ∼ 1.9 times baseline or ≥0.3 mg/dl increase |
| 2 | 2.0 ∼ 2.9 times baseline |
| 3 | ≥3.0 times baseline or increase in sCr to ≥4.0 mg/dl or initiation of renal replacement therapy |
Demographic and operative data previously reported to be associated with postoperative renal dysfunction and prognosis after aortic dissection were collected during retrospective chart review. Demographic variables included age, gender, hemodynamic status at presentation (blood pressure, heart rate, and the presence of shock or cardiac tamponade), body mass index (BMI), history of smoking, history of hypertension, diabetes mellitus, dyslipidemia, peripheral vascular disease, coronary artery disease, previous cardiothoracic surgery or atrial fibrillation, and contrast-enhanced computed tomography findings suggestive of visceral or limb malperfusion. Organ malperfusion was defined by the finding of vascular occlusion or extensive compression with reduced contrast enhancement of the dependent vascular territory. Operative variables recorded included the time that elapsed between symptom onset and surgery, extracorporeal circulation time, and the occurrence of postoperative infection. We also reviewed routine laboratory data collected before admission and during hospitalization (every day within first week after operation and then at least twice a week until discharge). Occurrences of mortality and major adverse cardiovascular and cerebrovascular events (MACCEs, which include myocardial infarction, cerebrovascular events, and death) were recorded from our institution’s electronic health record system. For those patients who were not followed up in our hospital, we undertook a telephone survey to obtain recent information.
We used Kaplan-Meier methods and multivariate Cox proportional hazards regression to assess the impact of AKI (stages 0 to 3) for both mortality and MACCE. The parameter used in multivariate analysis included major clinical factors that are known to affect the outcome of TA-AAD and clinical factors that are known to increase postoperative AKI to control confounding bias. Hazard ratios were calculated using the Cox proportional hazards model after adjustment for potentially confounding factors. As AKI was defined using peak sCr concentration before the seventh postoperative day, we defined 7 days after the hospitalization as the origin time for the survival analysis. We also examined the association between risk factors and AKI using logistic regression modeling.
All analyses were carried out using SAS software package, version 9.4 (SAS Institute, Cary, North Carolina). Continuous variables are expressed as the mean with SD or the median with the interquartile range, and categorical variables as frequencies and proportions (percentages).
Results
The patients’ clinical characteristics and operative data are presented in Table 2 . The median follow-up time was 943 days. The mean age of the patients was 66.4 years (range 59 to 94 years), and 193 patients (52%) were >70 years. The most common symptoms on presentation included chest pain (70%), back pain (56%), and syncope (12%). Emergent surgery was performed on the ascending aorta (62%), aortic arch (37%), or aortic valve (12%); 26 patients (7%) underwent re-thoracotomy because of either infection or hemorrhage.
| Variables | Overall(n=375) | Acute Kidney Injury | p -Value | |
|---|---|---|---|---|
| YES (n=165) | NO (n=210) | |||
| Age, mean ± SD (years) | 66.4 ± 13.3 | 65.5 ± 13.4 | 67.0 ± 13.3 | 0.28 |
| Male | 196 (52%) | 99 (60%) | 97 (46%) | 0.0073 |
| BMI, mean ± SD (kg/m 2 ) | 23.2 ± 3.9 | 24.0 ± 4.0 | 22.6 ± 3.6 | 0.0003 |
| Smoker | 97 (26%) | 47 (29%) | 50 (24%) | 0.30 |
| Hypertension | 303 (81%) | 134 (81%) | 169 (81%) | 0.86 |
| Diabetes mellitus | 25 (7%) | 8 (5%) | 17 (8%) | 0.21 |
| Obesity | 19 (5%) | 11 (7%) | 8 (5%) | 0.21 |
| Peripheral vascular disease | 4 (1.1%) | 1 (0.6%) | 3 (1.4%) | 0.79 |
| Coronary artery disease | 14 (4%) | 6 (4%) | 8 (4%) | 0.93 |
| Cerebrovascular disease | 33 (9%) | 14 (9%) | 19 (9%) | 0.84 |
| Previous cardiac surgery | 22 (6%) | 12 (7%) | 10 (5%) | 0.32 |
| Shock or tamponade (Systolic BP<80mmHg) | 50 (13%) | 23 (14%) | 27 (13%) | 0.068 |
| Coronary dissection | 19 (5%) | 14 (9%) | 5 (2%) | 0.0075 |
| Congestive heart failure | 14 (4%) | 5 (3%) | 9 (4%) | 0.52 |
| Renal malperfusion | 26 (7%) | 22 (13%) | 4 (2%) | <0.0001 |
| Lower limb malperfusion | 27 (7%) | 14 (9%) | 13(6%) | 0.39 |
| Mecentric malperfusion | 6 (2%) | 5 (3%) | 1 (0.5%) | 0.12 |
| Neurologic dysfunction | 20 (5%) | 10 (6%) | 10 (5%) | 0.58 |
| Left ventricular ejection fraction <50% | 22 (6%) | 13 (8%) | 9 (4%) | 0.14 |
| Atrial fibrillation | 17 (5%) | 9 (6%) | 8 (4%) | 0.45 |
| White blood cell count, mean ± SD(/μl) | 12714 ± 4866.5 | 12936.4 ± 5084 | 12540 ± 4693 | 0.43 |
| Hemoglobin, mean ± SD(g/dL) | 12.5 ± 1.9 | 12.6 ± 2.2 | 12.5 ± 1.7 | 0.63 |
| Platelet count, mean ± SD(x10 4 μl) | 18.2 ± 6.2 | 18.1 ± 7.0 | 18.2 ± 5.4 | 0.92 |
| Glucose, mean ± SD(mg/dl) | 157.2 ± 56.0 | 160.3 ± 55.8 | 154.8 ±56.2 | 0.35 |
| C reactive protein (CRP), mean ± SD(mg/dl) | 1.8 ± 4.4 | 2.1 ± 4.9 | 1.6 ± 4.0 | 0.25 |
| Peak CRP, mean ± SD(mg/dl) | 13.0 ± 6.2 | 14.6 ± 6.7 | 11.7 ± 5.4 | <0.0001 |
| estimated glomerular filtration rate, mean ± SD(ml/min/1.73 cm 2 ) | 63.1 ± 21.8 | 61.4 ± 21.6 | 64.4 ± 21.9 | 0.19 |
| Uremic acid, mean ± SD(mg/dl) | 5.8 ± 1.7 | 5.9 ± 1.8 | 5.7 ± 1.7 | 0.22 |
| Onset to operation time, median (min, max) (hours) | 6 (2, 170) | 6 (2.0, 170) | 6 (2, 168) | 0.56 |
| Extracorporeal circulation time, mean ± SD (minutes) | 139.5 ± 54.4 | 151.7 ± 61.8 | 129.9 ± 45.7 | 0.0001 |
AKI was observed in 165 patients within 7 days of surgery (44%): 89 patients (24%) fulfilled the KDIGO criteria for stage 1 AKI, 23 patients (6%) for stage 2, and 53 patients (14%) for stage 3. Postoperative outcomes and major complications are summarized in Table 3 . In total, 33 patients (9%) required temporary renal replacement therapy (dialysis) after operation and 11 (3%) progressed to dialysis-dependent end-stage renal disease. The mean length of hospital stay and intensive care unit stay was significantly longer in those who developed AKI.
| Variables | Acute Kidney Injury | p -Value | |
|---|---|---|---|
| YES (n=165) | No (n=210) | ||
| Hospital stay (days), median (min, max) | 22(4, 229) | 20 (2, 143) | 0.037 |
| Intensive care unit stay (days), median (min, max) | 2 (1, 83) | 2 (1, 26) | <0.0001 |
| Ventilation (days), median (min, max) | 1 (0, 63) | 1 (0, 12) | <0.0001 |
| Tracheostomy creation | 8 (5%) | 2 (1%) | 0.045 |
| Temporary dialysis | 31 (19%) | 2 (1%) | <0.0001 |
| End-stage renal disease | 11 (7%) | 0 | 0.0005 |
| Sepsis | 29 (18%) | 9 (4%) | <0.0001 |
| Inhospital myocardial infarction | 3 (2%) | 0 | 0.17 |
| Inhospital stroke | 24 (15%) | 15 (7%) | 0.020 |
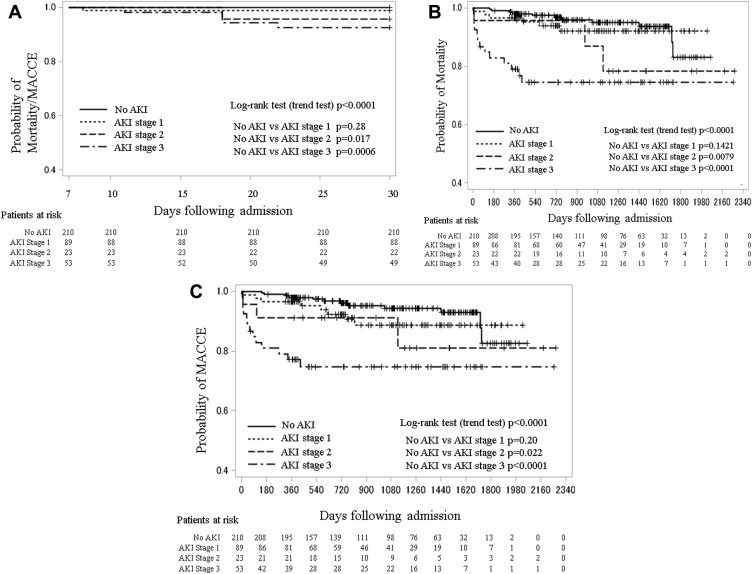
The overall 30-day and long-term mortality was 2.6% and 8.8%, respectively. Figure 1 shows Kaplan-Meier survival curves of both 30-day/long-term mortality and 30-day/long-term occurrences of MACCE. Both mortality and MACCE correlated significantly with the severity of AKI defined by the KDIGO criteria. Multivariate Cox analysis for mortality ( Table 4 ) and MACCE ( Table 5 ) revealed that AKI stage 3 was a significant and independent risk factor after adjustment for other major clinical factors, including those known to increase postoperative AKI, eliminating the possibility that AKI itself was a confounding factor. There was no significant association between the earlier stages of AKI and mortality or MACCE. Other major risk factors predicting poor outcome were age and elevated peak perioperative serum C-reactive protein concentration. High hazard ratios were also found for coronary dissection before surgery, low BMI, reduced preoperative left ventricular ejection fraction, and neurologic dysfunction at the time of admission, but these did not reach statistical significance.
| Hazard ratio (95% CI) | p -Value | |
|---|---|---|
| Sex (female) | 1.08 (0.48-2.44) | 0.86 |
| Age in 10-year increments | 1.79 (1.18-2.72) | 0.0067 |
| Body mass index in 5 increments | 0.75 (0.43-1.28) | 0.29 |
| Smoker | 0.52 (0.17-1.59) | 0.25 |
| Hypertension | 4.19 (1.03-17.06) | 0.05 |
| Diabetes | 1.56 (0.42-5.79) | 0.50 |
| Coronary artery disease | 1.40 (0.29-6.88) | 0.68 |
| Neurologic dysfunction | 2.59 (0.75-8.90) | 0.131 |
| Malperfusion | 0.63 (0.20-1.99) | 0.43 |
| Atrial fibrillation | 1.64 (0.41-6.51) | 0.48 |
| Congestive heart failure | 0.81 (0.11-6.15) | 0.84 |
| Coronary dissection | 3.51 (0.72-17.19) | 0.12 |
| Reduced preoperative left ventricular ejection fraction | 1.53 (0.39-6.01) | 0.54 |
| Extracorporeal circulation time | 1.03 (0.96-1.11) | 0.42 |
| Acute kidney injury stage 1 | 1.61 (0.55-4.68) | 0.39 |
| Acute kidney injury stage 2 | 1.67 (0.40-6.98) | 0.48 |
| Acute kidney injury stage 3 | 6.83 (2.52-18.52) | 0.00020 |
| Perioperative peak C reactive protein | 1.08 (1.01-1.15) | 0.016 |
| Perioperative sepsis | 0.62 (0.19-2.02) | 0.43 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


