Although coffee is a widely used, pharmacologically active beverage, its impact on the cardiovascular system is controversial. To explore the effect of acute caffeine ingestion on brachial artery flow-mediated dilation (FMD) in subjects without coronary artery disease (CAD; controls) and patients with CAD, we prospectively assessed brachial artery FMD in 40 controls and 40 age- and gender-matched patients with documented stable CAD on 2 separate mornings 1 week to 2 weeks apart. After overnight fasting, discontinuation of all medications for ≥12 hours, and absence of caffeine for >48 hours, participants received capsules with caffeine 200 mg or placebo. One hour after drug ingestion, participants underwent brachial artery FMD and nitroglycerin-mediated dilation (NTG) using high-resolution ultrasound. As expected, patients with CAD were more oftein diabetic, hypertensive, obese, dyslipidemic, and smoked more than controls (p <0.01 for all comparisons). Aspirin, Clopidogrel, angiotensin-converting enzyme inhibitors, β blockers, and statins were significantly more common in patients with CAD than in controls (p <0.01 for all comparisons). At baseline, FMD, but not NTG, was significantly lower in patients with CAD compared to controls. Acute caffeine ingestion significantly increased FMD (patients with CAD 5.6 ± 5.0% vs 14.6 ± 5.0%, controls 8.4 ± 2.9% vs 18.6 ± 6.8%, p <0.001 for all comparisons) but not NTG (patients with CAD 13.0 ± 5.2% vs 13.8 ± 6.1%, controls 12.9 ± 3.9% vs 13.9 ± 5.8%, p = NS for all comparisons) and significantly decreased high-sensitivity C-reactive protein (patients with CAD 2.6 ± 1.4 vs 1.4 ± 1.2 mg/L, controls 3.4 ± 3.0 vs 1.2 ± 1.0 mg/L, p <0.001 for all comparisons) in the 2 groups compared to placebo. In conclusion, acute caffeine ingestion significantly improved endothelial function assessed by brachial artery FMD in subjects with and without CAD and was associated with lower plasma markers of inflammation.
Although coffee is 1 of the most widely used, pharmacologically active beverages, its impact on the cardiovascular system is controversial. Although data regarding its effect on endothelial function are limited, several studies have found that coffee consumption is associated with impairment of flow-mediated dilation (FMD) of the brachial artery, aortic stiffness, and wave reflections. Others have maintained that neither caffeinated nor decaffeinated filtered coffee has a detrimental effect on markers of endothelial function, whereas the ATTICA study found that moderate coffee consumption is related to increased plasma concentrations of general inflammation markers. We aimed therefore to explore the effect of acute caffeine ingestion on brachial FMD in subjects without coronary artery disease (CAD) and in patients with stable CAD.
Methods
In a randomized, double-blind, placebo-controlled, cross-over study, we prospectively assessed brachial artery FMD in 80 consecutive subjects recruited from the endothelial function assessment laboratory of the Leviev Heart Center at the Sheba Medical Center (Tel Hashomer, Israel; 40 volunteers without and 40 age- and gender-matched patients with documented stable CAD) on 2 separate mornings 1 week to 2 weeks apart.
Volunteers without CAD were defined as subjects without a history of chest pain or myocardial infarction, coronary artery bypass grafting surgery, coronary angiography with angioplasty and/or stenting, cerebrovascular accident, or peripheral vascular disease with normal electrocardiogram and echocardiogram on admission. All patients without CAD were referred by primary care physicians or through direct patient request for risk factor evaluation through a primary prevention clinic. No patient was referred because of chest pain. Primary care physicians send patients to the assessment laboratory for CAD assessment (low vs high) to help them decide on treatment management of patients with risk factors for CAD. All patients underwent a full consultation by a cardiologist who performed primary prevention risk management according to updated National Cholesterol Education Program Adult Treatment Panel III and American Heart Association/American College of Cardiology/European Society of Cardiology guidelines. Patients with stable CAD were defined as those with a >6-month history of myocardial infarction and coronary artery bypass grafting surgery or coronary angiography with angioplasty or stenting >6 months previously. Exclusion criteria included atrial fibrillation, sinus bradycardia (heart rate <50 beats/min) without a pacemaker, sick sinus syndrome, second- or third-degree atrioventricular block, intolerance to nitrates, renal failure with serum creatinine >1.5 mg/dl, New York Heart Association class >II heart failure, history of drug or alcohol abuse, chronic liver disease, or refusal to sign the informed consent form.
After an overnight fast, discontinuation of all medications for ≥12 hours, absence of caffeine ingestion for >48 hours (including coffee, tea, cola, alcohol and flavonoid-containing beverages, and energy supplements), >48-hour cessation of cigarette smoking, and response to a specifically designed dietary questionnaire (for caffeine estimation), participants received capsules containing caffeine 200 mg (80-mg caffeine ingestion = 1 cup of coffee) or placebo administered at 7.00 a.m. with tap water 250 ml. Distribution was in a rotating randomized fashion, such that each subject received caffeine or placebo capsules once throughout the study period. Before (baseline) and 1 hour after study drug ingestion, participants underwent brachial artery FMD and nitroglycerin-mediated dilation (NTG) using high-resolution ultrasound. Electrocardiographic ad echocardiographic assessments and blood tests for serum caffeine levels, lipids, complete blood cell count, electrolytes, fasting glucose, homocysteine, adiponectin, and high-sensitivity C-reactive protein (hs-CRP) were performed.
Blood samples were centrifuged immediately for 15 minutes at 3,000/min. Sera were stored at −20°C and tested at the end of the study. Serum adiponectin levels were assessed using an enzyme-linked immunosorbent assay as described previously. Serum caffeine was assayed by an enzyme-multiplied immunoassay technique (Olympus AU2700, Olympus America, Inc., Center Valley, Pennsylvania). The hospital review board approved the study, and all participants gave written informed consent ( http://www.clinicaltrials.gov , identifier NCT00564824 ).
Caffeine 200 mg and placebo capsules were manufactured by the Sheba Medical Center pharmacy and the therapy sequence for each patient (caffeine at first visit, placebo at second visit, or vice versa) was prepared before study initiation by computer-generated randomization software. Investigators, coordinators, and patients were blinded to the drugs throughout the study. All capsule codes were kept locked at the Sheba Medical Center pharmacy throughout the study period and delivered to the study statistician only at closeout.
Endothelial function in the form of endothelium-dependent brachial artery FMD was measured as previously described using a 15- to 6-MHz linear array (15-6L HP) ultrasound system (HP SONOS 7500 cv System, Agilent Technologies, Inc., Andover, Massachusetts). A pneumatic tourniquet (Hokanson, AG101, Bellevue, Washington, DC) placed around the left upper arm proximal to the target artery (upper-arm occlusion) was inflated after the baseline phase to 50 mm Hg above the subject’s systolic blood pressure and held for 5 minutes. Diameter and Doppler flow velocity were measured at baseline and immediately after cuff deflation at 20, 40, 60, 90, and 120 seconds.
A second 2-minute baseline scan at rest was recorded to confirm vessel recovery 13 minutes after cuff deflation. Scanning was performed continuously for 5 minutes after administration of a sublingual nitroglycerin tablet (0.4 mg, Nitrostat, Park-Davis, New York, New York).
Ultrasound images were recorded on an S-VHS videotape with a SLV-RS7 videocassette recorder (SONY, California). Brachial artery diameter was measured from the anterior to the posterior interface between the media and adventitia (“m line”) at a fixed distance. Mean diameter was calculated from 4 cardiac cycles synchronized with R-wave peaks on electrocardiogram. All measurements were calculated at end-diastole to avoid possible errors resulting from variable arterial compliance. Internal diameter was calculated with PC Prosound (University of Southern California, Los Angeles, California) using a Horita data translation image processing board (DT2862-60Hz; Horita, Mission Viejo, California). FMD and NTG were expressed as percent change compared to those at the initial scan at rest. FMD was computed from the formula ([maximum diameter minus baseline diameter]/baseline diameter × 100). Percent FMD using maximal brachial artery diameter achieved after cuff deflation was used as an index of endothelium-dependent dilation; percent dilatation obtained 5 minutes after administration of nitroglycerin represented percent NTG. FMD and NTG vasodilatory response measurements were carried out in blind fashion. Intraobserver correlation coefficients for baseline and deflation diameters were 0.99. Absolute errors between measurements were 0 to 0.12 mm (for brachial artery diameter) and 0.02% to 2.98% (for FMD). Determination of endothelial function was performed in accordance with published guidelines.
A power calculation assessment based on the effect of caffeine on endothelial function in healthy subjects was performed before the study. Assuming a standardized difference of 0.72, a p value of 0.05, and a power of 85%, the number per group (healthy subjects and those with CAD) was estimated to be 30. Accounting for a 2-week 5% drop-out rate, we estimated that the total number of enrolled subjects should be 72. Values are expressed as mean ± SD for continuous variables and frequency and percentage for categorical variables. Distributions of continuous variables were assessed using Kolmogorov–Smirnov test. Comparisons between controls and patients with CAD were assessed using independent t test for continuous variables and chi-square test for categorical variables. Changes within caffeine and placebo treatments and differences between healthy subjects and those with CAD were calculated by repeated analysis measurements, which produced 3 tests of significance: difference in treatment changes between groups (interaction between treatment and group), total changes between treatments, and differences between groups in general. Correlations between serum caffeine levels and other continuous variables were assessed using Pearson or Spearman correlation test. Multivariate regression analysis was performed to predict percent FMD by independent variables. All statistical calculations were performed using SPSS 17.0 (SPSS, Inc., Chicago, Illinois). A p value <0.05 represented statistical significance.
Results
The cohort consisted of 80 consecutive subjects, of whom 40 had stable CAD and 40 were without established cardiovascular disease (controls). Table 1 presents baseline characteristics and clinical features of the entire study population. As expected, all CAD risk factors were more common in patients with CAD. In addition, aspirin, clopidogrel, β blockers, angiotensin-converting-enzyme inhibitors, and statin administration were more common in patients with CAD whose left ventricular ejection fraction was significantly lower compared to controls.
| Variable | Controls | Patients With CAD | p Value |
|---|---|---|---|
| (n = 40) | (n = 40) | ||
| Age (years) | 53 ± 6 | 53 ± 8 | 0.846 |
| Men | 33 (83%) | 33 (83%) | 0.981 |
| Smokers | 2 (5%) | 8 (20%) | 0.043 |
| Diabetes mellitus | 0 | 11 (28%) | <0.001 |
| Hypertension | 1 (3%) | 21 (53%) | <0.001 |
| Body mass index >25 kg/m 2 | 4 (10%) | 32 (80%) | <0.001 |
| Hypercholesterolemia (>200 mg/dl) | 2 (5%) | 40 (100%) | <0.001 |
| Family history of coronary artery disease | 10 (25%) | 19 (48%) | 0.030 |
| Concomitant medications | |||
| Aspirin | 1 (3%) | 40 (100%) | <0.001 |
| Clopidogrel | 0 | 10 (25%) | 0.001 |
| β Blockers | 1 (3%) | 28 (70%) | <0.001 |
| Calcium channel blockers | 0 | 2 (5%) | 0.246 |
| Angiotensin-converting enzyme inhibitors | 0 | 24 (60%) | <0.001 |
| Diuretics | 0 | 4 (10%) | 0.057 |
| Statins | 2 (5%) | 38 (95%) | <0.001 |
| Vitamins | 2 (5%) | 4 (10%) | 0.338 |
| Physical examination | |||
| Heart rate at rest (beats/min) | 69 ± 13 | 65 ± 11 | 0.211 |
| Systolic blood pressure (mm Hg) | 127 ± 17 | 128 ± 17 | 0.912 |
| Diastolic blood pressure (mm Hg) | 78 ± 10 | 78 ± 10 | 0.818 |
| Weight (kg) | 76 ± 18 | 85 ± 14 | 0.141 |
| Height (m) | 1.70 ± 0.10 | 1.76 ± 0.09 | 0.024 |
| Waist circumference (cm) | 92 ± 15 | 98 ± 15 | 0.143 |
| Body mass index (kg/m 2 ) | 26 ± 5 | 28 ± 4 | 0.418 |
| Blood tests | |||
| White blood cell count (number/μl) | 6,260 ± 1,825 | 7,176 ± 1,519 | 0.018 |
| Hemoglobin (g/dl) | 13.8 ± 2.4 | 14.1 ± 1.1 | 0.531 |
| Platelet count (number/μl) | 219,780 ± 48,902 | 209,230 ± 49,958 | 0.346 |
| Potassium (mEq/L) | 4.2 ± 0.5 | 4.3 ± 0.3 | 0.245 |
| Chloride (mEq/L) | 105 ± 2 | 105 ± 2 | 0.918 |
| Sodium (mEq/L) | 139 ± 2 | 139 ± 2 | 0.821 |
| Creatinine (mg/dl) | 0.95 ± 0.15 | 1.01 ± 0.13 | 0.074 |
| Urea (mg/dl) | 30 ± 8 | 34 ± 6 | 0.006 |
| Glucose (mg/dl) ⁎ | 89 ± 13 | 95 ± 19 | 0.130 |
| Total cholesterol (mg/dl) | 188 ± 32 | 137 ± 22 | <0.001 |
| Low-density lipoprotein cholesterol (mg/dl) | 114 ± 24 | 84 ± 17 | <0.001 |
| Triglycerides (mg/dl) | 107 ± 50 | 123 ± 57 | 0.207 |
| High-density lipoprotein cholesterol (mg/dl) | 51 ± 14 | 38 ± 7 | <0.001 |
| Homocysteine (μmol/L) | 12 ± 7 | 14 ± 7 | 0.354 |
| High-sensitivity C-reactive protein (mg/L) | 3.4 ± 3.0 | 2.6 ± 1.4 | 0.345 |
| Adiponectin (μmol/L) | 11.0 ± 6.5 | 7.4 ± 4.2 | 0.428 |
| Left ventricular ejection fraction (%) | 60 ± 5 | 46 ± 11 | <0.001 |
| Endothelial function | |||
| Baseline brachial artery diameter (mm) | 5.1 ± 1.2 | 5.8 ± 0.8 | <0.01 |
| Percent flow-mediated dilation † | 8.4 ± 2.9 | 5.6 ± 5.0 | <0.001 |
| Percent nitroglycerin-mediated dilation ‡ | 12.9 ± 3.9 | 13.0 ± 5.2 | 0.535 |
⁎ Blood glucose levels were measured after a 12-hour fast.
† Percent brachial artery diameter added by endothelium-dependent vasodilation after cuff deflation.
‡ Percent brachial artery diameter added by smooth muscle-dependent vasodilation after nitroglycerin administration.
As previously demonstrated, baseline brachial artery diameter was larger in the CAD group ( Table 1 ), whereas FMD was significantly greater and NTG was similar in subjects without CAD compared to patients with CAD. No adverse effects were noted during brachial artery testing of the 2 groups.
Although acute caffeine ingestion significantly improved FMD in patients with CAD and those without CAD, improvement was significantly greater in subjects without CAD compared to those with CAD ( Figure 1 , Table 2 ). Acute caffeine ingestion, however, did not significantly change NTG in the 2 study groups. Although acute caffeine ingestion significantly decreased hs-CRP in subjects without and those with CAD, its impact on control subjects without CAD was significantly higher compared to patients with CAD ( Figure 1 , Table 2 ). Baseline serum adiponectin was higher, although not statistically significant, in controls compared to patients with CAD ( Figure 1 , Table 1 ). Adiponectin, however, although increased by acute caffeine ingestion in the 2 study groups, reached statistical significance only in patients with CAD compared to controls ( Figure 1 , Table 2 ).
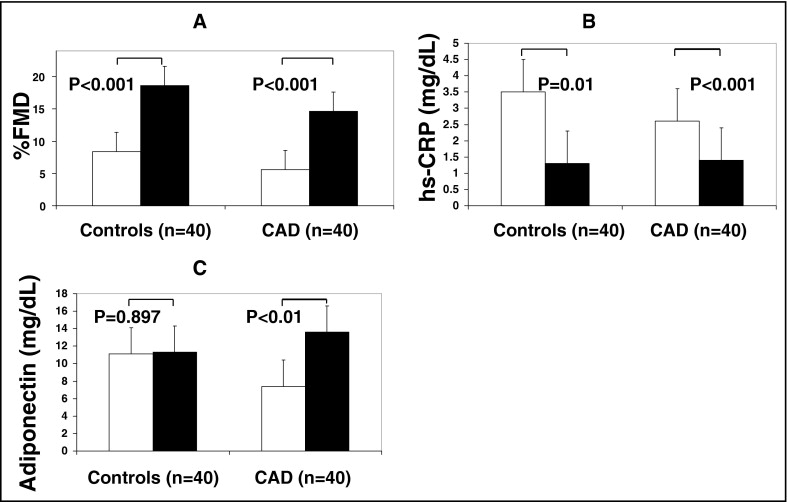
| Controls | Patients With CAD | p Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| (n = 40) | (n = 40) | Interaction | Group | Caffeine | |||||
| Placebo | Caffeine | p Value | Placebo | Caffeine | p Value | ||||
| Percent flow-mediated dilation ⁎ | 8.4 ± 2.9 | 18.6 ± 6.8 | <0.001 | 5.6 ± 5.0 | 14.6 ± 5.0 | <0.001 | 0.625 | 0.007 | <0.001 |
| Percent nitroglycerin-mediated dilation † | 12.9 ± 3.9 | 13.9 ± 5.8 | 0.768 | 13.0 ± 5.2 | 13.8 ± 6.1 | 0.867 | 0.458 | 0.426 | 0.623 |
| High-sensitivity C-reactive protein (mg/dl) | 3.4 ± 3.0 | 1.2 ± 1.0 | 0.012 | 2.6 ± 1.4 | 1.4 ± 0.009 | 0.009 | 0.197 | 0.393 | <0.001 |
| Adiponectin (mg/dl) | 11.0 ± 4.5 | 11.3 ± 7.5 | 0.897 | 7.4 ± 4.2 | 13.6 ± 5.9 | 0.001 | 0.035 | 0.614 | 0.021 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


