An increased prevalence of intrapulmonary right-to-left shunt (RLS) has been shown in patients with migraine. The aim of this study was to determine whether the size of intrapulmonary RLS was associated with migraine with aura (MA+) and migraine without aura (MA−) in subjects screened for hereditary hemorrhagic telangiectasia. A total of 462 consecutive subjects were screened for hereditary hemorrhagic telangiectasia and underwent transthoracic contrast echocardiography. A pulmonary shunt was established when contrast appeared in the left atrium after 4 cardiac cycles. Shunt size was assessed semiquantitatively as small (<30 microbubbles), moderate (30 to 100 microbubbles), or large (>100 microbubbles). A headache questionnaire was completed by 420 subjects (91%). Two independent neurologists diagnosed migraine according to the International Headache Society criteria. Of 420 screened subjects (mean age 43.4 ± 15.4 years, 61.4% women), 44 (10.5%) had MA+ and 45 (10.7%) had MA−. MA+ was an independent predictor for an intrapulmonary RLS (odds ratio [OR] 2.96, 95% confidence interval [CI] 1.36 to 6.47, p = 0.006) in multivariate analysis. MA− was not correlated with RLS (OR 1.21, 95% CI 0.56 to 2.64, p = 0.60). When comparing patients with MA+ to those without migraine in a multivariate analysis, the presence of an intrapulmonary shunt predicted MA+ (OR 2.5, 95% CI 1.2 to 5.2, p = 0.01), as did female gender (OR 3.15, 95% CI 1.29 to 7.65, p <0.01). The correlation of MA+ and RLS could be entirely attributed to large intrapulmonary shunts (OR 7.61, 95% CI 3.11 to 18.61, p <0.001), as small (OR 0.6, 95% CI 0.13 to 2.78, p = 0.52) and moderate (OR 1.33, 95% CI 0.35 to 5.02, p = 0.68) shunts did not appear to be risk factors for MA+. In conclusion, patients with large intrapulmonary RLS have an increased risk for MA+.
The existence of a true relation between right-to-left shunt (RLS) and migraine with aura (MA+) is controversial. The RLS-migraine hypothesis would be strengthened if a “dose-response” relation appears to be present. This has never been evaluated for intrapulmonary RLS. Intrapulmonary shunting through pulmonary arteriovenous malformations (PAVMs) is common in patients with hereditary hemorrhagic telangiectasia (HHT), an autosomal dominant inherited vascular disorder. Therefore, we prospectively studied the association of intrapulmonary shunt size, as documented semiquantitatively by transthoracic contrast echocardiography (TTCE), with migraine in a large group of subjects screened for possible HHT.
Methods
From May 2004 to May 2009, 492 consecutive subjects were screened for possible HHT at St. Antonius Hospital in The Netherlands. Three hundred fifty subjects were screened as first-degree family members of index patients with HHT, 9 subjects as second-degree family members, and 2 subjects as third-degree family members, and in 59 subjects, there was no positive family history for HHT (the latter group consisted of subjects with symptoms suggesting HHT or in whom PAVMs were detected on chest computed tomography). All subjects provided informed consent, and the study was approved by the hospital review board.
The screening protocol included systematic history and physical examination by a pulmonologist specializing in HHT. Screening for PAVMs was performed with high-resolution chest computed tomography and TTCE on the same day. Blood gas analysis was also routinely performed in all subjects. The clinical diagnosis of HHT was established according to the Curaçao criteria. These criteria consist of spontaneous and recurrent epistaxis, telangiectas at characteristic sites, visceral arteriovenous malformations or telangiectases, and a first-degree relative with HHT. Three criteria suffice for a definitive diagnosis of HHT, 2 criteria are considered as possible HHT, and no or 1 criterion renders the diagnosis unlikely. Genetic testing for the HHT-causing family mutation was offered to all screened subjects and performed as previously described. The diagnosis of HHT relied on the results of genetic testing and, when these were not available, on clinical judgment according to the diagnostic criteria.
Contrast echocardiography was performed as previously described. Briefly, the patient was positioned in the left lateral decubitus position and 10 ml agitated saline was injected while projecting the 4-chamber apical view without a Valsalva maneuver. A Valsalva maneuver was not performed because the objective of the TTCE was to screen for permanent intrapulmonary RLS in patients with HHT. TTCE was performed by 3 experienced echocardiographers. The results of TTCE were considered positive for a pulmonary RLS if microbubbles appeared in the left atrium after 4 cardiac cycles. When contrast was present in the left atrium within <4 cardiac cycles, this was considered a patent foramen ovale (27 patients in the study, 6% of 420 study patients). The results were scored as positive or negative for a pulmonary shunt or inappropriate for interpretation because of poor quality (5 patients in the present study). If the difference between an intracardiac or pulmonary shunt was not clear, the results of TTCE were assessed as positive for a pulmonary shunt. Opacification of the left ventricle was graded 1 (maximum of 30 microbubbles in left ventricle), 2 (30 to 100 microbubbles in left ventricle), and 3 (>100 microbubbles in left ventricle). This division was based on the maximum number of microbubbles counted in 1 still frame.
A structured headache questionnaire was sent to all patients before the screening visit in the outpatient clinic. Patients were asked about the presence, frequency, severity, duration, type, and site of headache. Furthermore, they were asked about the occurrence of scotoma, paresthesia, paresis, aphasia and aggravating factors, accompanying symptoms such as nausea, vomiting, phonophobia and/or photophobia, and if they were able to continue daily activities. The relative frequency of migraine attacks was reported by the patients as a score ranging from 0 (no headaches) to 6 (daily episodes). The severity of the headache attacks was scored on a scale ranging from 0 (no pain) to 10 (severe pain). The questions focused on the 6 months before the day of screening. Two independent neurologists, blinded to the other screening test results, reviewed the questionnaires and diagnosed migraine and MA+ according to the International Headache Society classification. Migraine was defined if ≥1 migraine attack occurred during the predefined period. When the 2 neurologists disagreed about the presence of aura in patients with migraine, these patients were classified as having MA+. When there was disagreement about the presence of migraine, patients were classified as having migraine.
Stroke and transient ischemic attack were diagnosed by a neurologist and in nearly all patients confirmed by computed tomography or magnetic resonance imaging of the brain. Screening for cerebral arteriovenous malformation was recommended to all patients with HHT, particularly so for those with HHT type 1, because the incidence of cerebral arteriovenous malformation in this patient group (15%) is higher than in patients with HHT type 2 (1%).
Descriptive statistics were used to describe patients and migraine characteristics. Differences between groups were analyzed using unpaired Student’s t tests for continuous variables and chi-square tests for nominal variables. Data are expressed either as mean ± SD or number (percentage). The level of significance was set at p <0.05. Univariate and multivariate statistical analyses with logistic regression were used to identify and estimate risk factors for pulmonary shunting and MA+ compared to subjects without migraine. After univariate analysis, variables with p values <0.10 were entered into a multivariate model. The comparison of the distribution of shunt grades between migraine categories was performed using the Mann-Whitney U test. Odds ratios (OR) and 95% confidence intervals (CIs) were calculated. Interobserver variability was evaluated by measuring the κ coefficient. Statistical analysis was performed using SPSS version 17.0.0 (SPSS, Inc., Chicago, Illinois).
Results
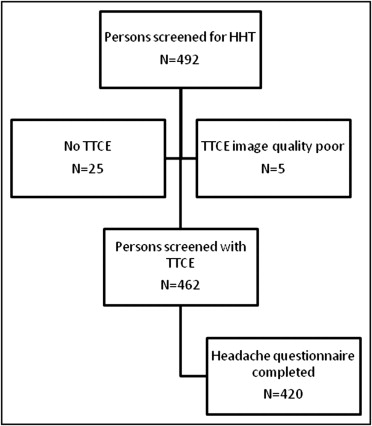
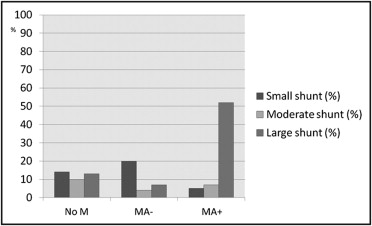
Of 492 consecutive subjects screened for HHT, TTCE with shunt grading was available in 462 ( Figure 1 ). Four hundred twenty subjects (91%; mean age 43.4 ± 15.4 years, 61% women) completed the headache questionnaire, and these were included in the study. The overall prevalence of migraine in the study participants was 21% and 10% for MA+ ( Table 1 ). Pulmonary shunts were present in 37% of subjects without migraine, 31% of those with migraine without aura, and 64% of those with MA+. Fifty-two percent of patients with MA+ displayed large intrapulmonary shunts. The distribution of different shunt grades differed significantly between patients with MA+ and those without migraine (Mann-Whitney U test, p <0.001) or those with migraine without aura (MA−) (p <0.001; Figure 2 ). Of 163 patients with pulmonary shunts, 141 (87%) had HHT, 11 (7%) had “possible” clinical diagnoses of HHT, and 11 (7%) did not have HHT. Baseline characteristics of patients with migraine and controls are listed in Table 1 .
| Variable | n | No Migraine | MA− | MA+ |
|---|---|---|---|---|
| Number of patients | 420 | 331 | 45 | 44 |
| Men/women | 162/258 | 148/183 | 6/39 | 8/36 |
| Age (years) | 43.4 ± 15.4 | 44.3 ± 15.6 | 40.9 ± 13.8 | 39.5 ± 14.6 |
| Oral contraceptive use | 52 (12%) | 34 (10%) | 8 (18%) | 10 (23%) |
| Platelet inhibitor | 10 (4%) | 1 (0.3%) | 1 (2%) | 8 (18%) |
| Stroke or transient ischemic attack | 13 (3%) | 10 (3%) | 0 | 3 (7%) |
| Cerebral arteriovenous malformation ⁎ | 9 (2%) | 7 (2%) | 1 (2%) | 1 (2%) |
| HHT † | 217 (52%) | 169 (51%) | 18 (40%) | 30 (68%) |
| No pulmonary shunt | 257 (61%) | 210 (63%) | 31 (69%) | 16 (36%) |
| Any pulmonary shunt size | 163 (39%) | 121 (37%) | 14 (31%) | 28 (64%) |
| Small pulmonary shunt | 58 (14%) | 47 (14%) | 9 (20%) | 2 (5%) |
| Moderate pulmonary shunt | 37 (9%) | 32 (10%) | 2 (4%) | 3 (7%) |
| Large pulmonary shunt | 68 (16%) | 42 (13%) | 3 (7%) | 23 (52%) |
⁎ Data were available for 101 patients.
† Definite HHT on the basis of confirmed genetic mutation or Curaçao clinical criteria (see “Methods”; unlikely = 0 or 1 criterion, possible = 2 criteria, definite = 3 criteria).
MA− was not associated with the presence of an intrapulmonary RLS (OR 1.21, 95% CI 0.56 to 2.64, p = 0.63). Conversely, MA+ was an independent predictor of the presence of a pulmonary shunt in univariate and multivariate analysis (OR 2.96, 95% CI 1.36 to 6.47, p = 0.006). Arterial oxygen tension showed a trend for an association with any pulmonary shunt after correction for other risk factors. These data are summarized in Table 2 . However, patients with large pulmonary shunts displayed significantly lower partial oxygen pressures (10.5 ± 1.8 kPa) compared to patients without shunts (12.2 ± 1.5 kPa, p <0.001), with small shunts (12.3 ± 1.4 kPa, p <0.001), or with moderate shunts (12.8 ± 1.5 kPa, p <0.001).
| Variable | Pulmonary Shunt on TTCE | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | ||
| Total | 163 | ||||
| Age (years) (mean ± SD) | 42.8 ± 15.4 | 1.00 (0.98–1.01) | 0.51 | ||
| Gender | |||||
| Female | 106 (65%) | 1.30 (0.86–1.93) | 0.23 | ||
| Male | 57 (35%) | ||||
| Medications | |||||
| β blockers | 12 (8%) | 1.26 (0.57–2.77) | 0.57 | ||
| Platelet inhibitors | 3 (2%) | 0.66 (0.17–2.60) | 0.56 | ||
| Oral contraceptives | 25 (16%) | 1.54 (0.86–2.77) | 0.15 | ||
| Neurologic event | |||||
| Stroke | 7 (4%) | 11.4 (1.40–94.25) | 0.02 | ||
| Transient ischemic attack | 2 (1%) | 1.05 (0.17–6.36) | 0.96 | ||
| Stroke or transient ischemic attack | 9 (6%) | 3.67 (1.11–12.13) | 0.03 | 2.03 (0.51–8.17) | 0.32 |
| Cerebral arteriovenous malformation ⁎ | 5 (8%) | 0.62 (0.18–2.80) | 0.70 | ||
| Migraine | |||||
| No migraine | 121 (74%) | Reference | Reference | ||
| MA− | 14 (9%) | 0.81 (0.41–1.58) | 0.53 | 1.21 (0.56–2.64) | 0.63 |
| MA+ | 28 (17%) | 3.03 (1.58–5.82) | 0.001 | 2.96 (1.36–6.47) | 0.006 |
| HHT † | |||||
| Unlikely | 12 (7%) | Reference | Reference | ||
| Possible | 11 (7%) | 3.25 (1.32–7.99) | 0.007 | 3.35 (1.34–8.39) | 0.01 |
| Definite | 141 (86%) | 14.8 (7.74–28.17) | <0.001 | 13.53 (7.00–26.17) | <0.001 |
| Partial oxygen tension (kPa) | 11.7 ± 1.9 | 0.81 (0.72–0.92) | 0.001 | 0.87 (0.76–1.00) | 0.06 |
| Hemoglobin (mmol/L) | 8.6 ± 1.4 | 0.95 (0.81–1.12) | 0.54 | ||
⁎ Data were available for 101 patients.
† Diagnosis was based on the 4 Curaçao clinical criteria (see text) (unlikely = 0 or 1 criterion, possible = 2 criteria, definite = 3 criteria).
When comparing patients with MA+ to those without migraine, the prevalence of intrapulmonary RLS was higher in the former group of patients (OR 2.53, 95% CI 1.23 to 5.18, p = 0.01). Moreover, a large intrapulmonary shunt was a powerful independent predictor of MA+ (OR 7.61, 95% CI 3.11 to 18.61, p <0.001), but small or moderate shunts did not appear to be risk factors for MA+. Apart from large shunts, only female gender was an independent predictor of MA+ (OR 3.15, 95% CI 1.29 to 7.65, p <0.01). Patients with MA+ showed a trend toward younger age, but this association was not sustained after correction. These data are listed in Table 3 .
| Variable | MA+ | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | ||
| Total | 44 | ||||
| Age (years) (mean ± SD) | 39.5 ± 14.6 | 0.98 (0.96–1.00) | 0.06 | 0.98 (0.95–1.00) | 0.08 |
| Gender | |||||
| Female | 36 (81.8) | 3.64 (1.64–8.07) | 0.001 | 3.15 (1.29–7.65) | 0.011 |
| Male | 8 (18.2) | ||||
| Medications | |||||
| β blockers | 2 (5%) | 0.64 (0.15–2.80) | 0.55 | ||
| Platelet inhibitors | 1 (2%) | 0.94 (0.12–7.69) | 0.95 | ||
| Oral contraceptives | 10 (23%) | 2.56 (1.16–5.64) | 0.02 | 1.21 (0.44–3.33) | 0.71 |
| Neurologic event | |||||
| Stroke | 2 (5%) | 2.58 (0.50–13.19) | 0.26 | ||
| Transient ischemic attack | 1 (2%) | 1.90 (0.21–17.40) | 0.57 | ||
| Stroke or transient ischemic attack | 1 (6%) | 0.56 (0.06–4.91) | 0.60 | ||
| Pulmonary shunt † | |||||
| None | 16 (36%) | Reference | Reference | ||
| Small | 2 (5%) | 0.56 (0.12–2.51) | 0.45 | 0.60 (0.13–2.78) | 0.52 |
| Moderate | 3 (7%) | 1.23 (0.34–4.46) | 0.75 | 1.33 (0.35–5.02) | 0.68 |
| Large | 23 (52%) | 7.19 (3.50–14.75) | <0.001 | 7.61 (3.11–18.61) | <0.001 |
| HHT | |||||
| Unlikely | 10 (23%) | Reference | |||
| Possible | 30 (68%) | 1.73 (0.81–3.67) | 0.15 | ||
| Definite | 4 (9%) | 1.32 (0.39–4.49) | 0.66 | ||
| Partial oxygen tension (kPa) | 11.5 ± 2.1 | 0.83 (0.69–0.99) | 0.04 | 1.00 (0.80–1.25) | 0.99 |
| Hemoglobin (mmol/L) | 8.8 ± 1.2 | 1.08 (0.82–1.40) | 0.59 | ||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


