The aim of the study was to evaluate the additional diagnostic value of real-time 3-dimensional transesophageal echocardiography (RT3D-TEE) for surgically recognized mitral valve (MV) prolapse anatomy compared to 2-dimensional transthoracic echocardiography (2D-TTE), 2D-transesophageal echocardiography (2D-TEE), and real-time 3D-transthoracic echocardiography (RT3D-TTE). We preoperatively analyzed 222 consecutive patients undergoing repair for prolapse-related mitral regurgitation using RT3D-TEE, 2D-TEE, RT3D-TTE, and 2D-TTE. Multiplanar reconstruction was added to volume-rendered RT3D-TEE for quantitative prolapse recognition. The echocardiographic data were compared to the surgical findings. Per-patient analysis of RT3D-TEE identified prolapse in 204 patients more accurately (92%) than 2D-TEE (78%), RT3D-TTE (80%), and 2D-TTE (54%). Even among those 60 patients with complex prolapse (>1 segment localization or commissural lesions), RT3D-TEE correctly identified 58 (96.5%) compared to 42 (70%), 31 (52%), and 21 (35%) detected by 2D-TEE, RT3D-TTE, and 2D-TTE (p < 0.0001). Multiplanar reconstruction enabled RT3D-TEE to differentiate dominant (≥5-mm displacement) and secondary (2 to <5-mm displacement) prolapsed segments in agreement with surgically recognized dominant lesions (100%), but with a low predictive value (34%) for secondary lesions. In addition, owing to the identification of clefts and subclefts (indentations of MV tissue that extended ≥50% or <50% of the total leaflet height, respectively), RT3D-TEE accurately characterized the MV anatomy, including that which deviated from the standard nomenclature. In conclusion, RT3D-TEE provided more accurate mapping of MV prolapse than 2D imaging and RT3D-TTE, adding quantitative recognition of dominant and secondary lesions and MV anatomy details.
Accurate localization of the valve lesion is crucial when planning the surgical repair strategy for prolapse-related mitral regurgitation. The recent availability of the MitraClip system further imposes a detailed depiction of mitral valve (MV) anatomy to predict and monitor percutaneous valve repair. The accuracy of 2-dimensional transthoracic echocardiography (2D-TTE) and transesophageal echocardiography (2D-TEE) can be affected by a nonsimultaneous and limited cutting plane that is unable to provide a comprehensive MV image, together with operator experience and a suboptimal acoustic window. The recent availability of real-time 3-dimensional transesophageal echocardiography (RT3D-TEE) might drive forward the diagnostic capability of ultrasound imaging, providing an immediate anatomic view of the MV similar to that seen by the surgeon. The aim of the present study was to evaluate the additional diagnostic value of RT3D-TEE compared to 2D imaging (2D-TTE and 2D-TEE) and RT3D-TTE for MV functional anatomy assessment, using as a standard the surgical findings in a group of patients with prolapse-related mitral regurgitation undergoing valve repair.
Methods
From May 2008 to January 2009, we prospectively enrolled 222 consecutive patients referred to our institute for surgical repair of prolapse-related mitral regurgitation. The inclusion criteria for the study were MV prolapse with related severe mitral regurgitation (effective regurgitant orifice ≥0.4 cm 2 , vena contracta >7 mm, regurgitant volume >60 ml); a complete transthoracic and transesophageal preoperative echocardiographic study; and surgical MV repair within 1 uneventful week from the ultrasound evaluation. To avoid potentially confounding factors on the MV prolapse appearance, we excluded those patients with associated valvular heart disease needing surgical correction (20 subjects), an unstable critically ill condition, or severe left ventricular pump dysfunction (ejection fraction <40%; 30 subjects). One additional subject was excluded because of a contraindication to TEE. The clinical and echocardiographic characteristics of the study population are listed in Table 1 .
| Characteristic | Value |
|---|---|
| Age (years) | 53 ± 14 |
| Women | 70 (31.5%) |
| Symptoms | 45 (20%) |
| Left ventricular end-diastolic diameter (mm) | 59 ± 5 |
| Left ventricular end-systolic diameter (mm) | 30 ± 18 |
| Left ventricular end-systolic diameter ≥40 mm | 48 (22%) |
| Left ventricular ejection fraction | 60 ± 10% |
| Left ventricular ejection fraction ≥60% | 200 (90%) |
| Left ventricular ejection fraction 40–59% | 22 (10%) |
| Left atrium diameter (mm) | 48 ± 5 |
| Left atrium volume (ml) | 70 ± 30 |
| Pulmonary artery hypertension ≥50 mm Hg | 60 (27%) |
| Atrial fibrillation | 50 (22.5%) |
Our institute’s ethics committee approved the study, and all subjects provided informed consent.
2D-TTE was performed on all patients using an iE33 (Philips Ultrasound, Andover, Massachusetts) with an S5 probe, using the standard approach. The MV segment evaluation followed the 6-segment model of the Carpentier nomenclature, with 2 additional segments for the MV commissures, according to the classification by Kumar et al. RT3D-TTE was done at the end of the 2D examination using the same machine with a matrix phased-array X3 probe. Full-volume and a wide sector RT3D zoom mode from both the parasternal and the apical window were performed. All 8 MV segments in every patient were subjectively classified as normal, prolapsing (displacement of leaflet tip ≥2 mm beyond the annular plane on the 2D images and systolic bright convexity or bulge on the 3D images), flail (eversion of the leaflet tip into the left atrium with evidence of ruptured related chordae tendineae), or noninterpretable. All images were acquired and stored in digital cine-loops.
2D and RT3D-TEE were performed using a Philips iE33 echocardiograph (Philips Ultrasound) connected to an X7-2t transesophageal probe. Bland sedation using midazolam was performed in only 20% of the patients. After introducing the probe into the esophagus, we performed 2D and color Doppler imaging using the standard approach. As with the imaging criteria for 2D-TTE, all 8-MV segments in every patient were classified as normal, prolapsing, or flail. RT3D-TEE was acquired from the midesophageal view using the full volume (3 to 7 beats) and the live 3D zoom mode in the long-axis view to include the MV, aorta, and left atrial appendage, providing a live “en face” surgical view of the MV from the left atrial perspective. Careful attention was paid when adapting the width and elevation of the zoom function over the region of interest with minimized sector width to improve temporal resolution (frame rate range 8 to 12 Hz) and when optimizing gain to visualize MV anatomy without dropout and artifacts. We also used the same 3D echocardiographic protocol for the subgroup of patients with atrial fibrillation. After capturing the 3D close-up MV image, we rotated it to obtain a surgical view from the atrium with the aorta in the foreground and the appendage on the left. Image quality was optimized on-line with a modifiable cutting plane to remove artifacts and obtain detailed visualization of MV segments.
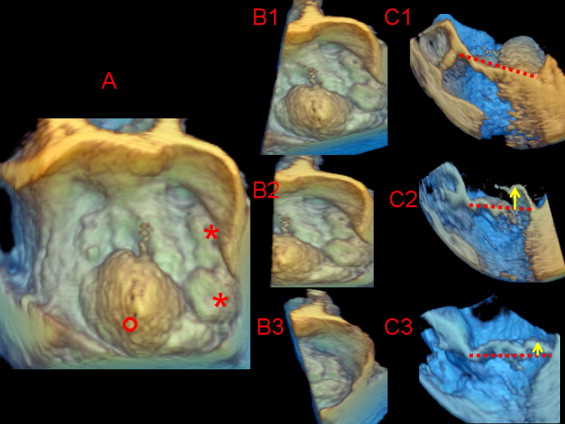
Each zoom-mode 3D data set was exported to a Cardio-View (Tomtec Imaging Systems, Munich, Germany) software workstation for image analysis and measurement. Analyzing the zoom mode 3D images, the observers could change the image orientation or cutting plane to obtain several cropped 2D tomographic images for multiplanar reconstruction of all MV segments on a longitudinal plane. Using the volume-rendered images, multiplanar reconstruction was used to optimize interpretation and quantify the displacement of the facing MV segments above the anteroposterior annular plane along the intercommissural coaptation surface. Full-volume data sets were excluded from the quantitative reanalysis owing to a substantial percentage (60%) of “stitch artifacts.” We identified the posterior leaflet segments using the commissures and clefts as referral points. The so-called posterior leaflet scallops were classified as lateral (P1), middle (P2), and medial (P3) starting from the anterolateral commissure to the first cleft, then the second cleft, and finally the posteromedial commissure, respectively. The definition of cleft and subcleft was determined from the measurement of indentation extension from the free margin to the leaflet base using the specific function incorporated in the Tomtec analysis. We accepted as a cleft an indentation of MV tissue extended over 50% of the total leaflet height. In contrast, an indentation of less than this cutoff was defined as a subcleft . The anterior leaflet segments were classified as lateral (A1), middle (A2), and medial (A3) using the facing segment of the posterior leaflet as the referral points. Prolapse was recognized when the tip of one or more valvular segments extended ≥2 mm relative to the leaflet-annular hinge points using a modifiable cutting plane to perform a multiplanar reconstruction long-axis view with a segment-by-segment analysis perpendicular to the intercommissural line. To avoid misidentification of the individual MV segments owing to the foreshortening effect, imaging plane cutting was appropriately oriented and adjusted segment-by-segment for the commissural landmarks. Prolapse localization was classified as one or more segment monoleaflet, bileaflet, commissural, or combined (leaflet and commissure). Furthermore, bileaflet prolapse was labeled as symmetric (facing segments) or asymmetric (nonfacing segments). In patients with multisegment lesions, the prolapsing segments were recognized as either dominant (maximal systolic coaptation displacement into the left atrium ≥5 mm) or secondary (maximal systolic coaptation displacement of 2 to <5 mm; Figure 1 ). Additional scallops exceeding the conventional Carpentier nomenclature were annotated. Echocardiographic examinations were performed by 2 clinicians (G.L.C., I.A.) with experience of >1,000 echocardiographic examinations and specific training in the interpretation and analysis of RT3D-TEE in 40 patients with MV prolapse. The same 2 clinicians performed random and blinded analyses of all alternative echocardiographic image modalities and surgical findings. The discrepancies between the 2 observers were resolved by reanalysis consensus to obtain a final interpretation of the echocardiographic findings.
The inter- and intraobserver variability in the echocardiographic diagnosis was assessed in a group of 50 patients randomly selected from the study population. In particular, the observer variability for RT3D-TEE in prolapse diagnosis and dominant or secondary lesion recognition was assessed by retesting the multiplanar reconstruction analysis and measurement of the segmental MV displacement above the annulus.
With cardiopulmonary bypass and cardioplegic arrest, the MV anatomy was independently assessed in every patient by 2 expert surgeons (≥500 mitral repairs performed). The modified Carpentier nomenclature was applied to depict the MV anatomy using the commissures as the referral points. To depict the MV anatomy, a scallop was identified as a segment of a leaflet between 2 clefts. The sighted intraleaflet indentations involving ≥50% of the leaflet length, together with recognition of related chordae insertion, were identified as clefts , and indentations below this cutoff were considered subclefts . The multiple middle scallops observed between P1 and P3 and the presence of ruptured chordae tendinee (flail) were annotated. Prolapsing segments were carefully identified in every patient by 2 expert surgeons using the anulus at the base of the anterior or posterior leaflets as the reference point for the leaflet free-edge coaptation. The prolapse height was evaluated with 2 hooks used to pull the anterior and posterior leaflet free edges. Dominant prolapsing lesions were identified by the clear displacement (≥5 mm) of the free edge of the MV segments above the annular reference point. Keeping the annular plane as a reference point, the remaining MV segments were subsequently compared and classified as a secondary prolapse (minor overriding compared to the annular plane and the dominant lesion) or normal (absence of annular overriding). Therefore, using the anatomic findings, the surgeons targeted the main repair strategy to the dominant lesions, adding minor procedures for the secondary lesions. Intraoperative routine evidence from TEE of successful targeting repair was also used as supportive diagnostic criteria for surgically recognized prolapsing segments. The surgeons were aware of the 2D echocardiographic diagnosis but were unaware of the 3D-specific information.
The quantitative variables with tested normal distribution and categorical variables are expressed as the mean ± SD and percentages, respectively. The sensitivity and specificity of the 2D and 3D images for the identification of both dominant and secondary prolapsed segments were calculated using the surgical findings as reference. To compensate for the intracluster correlation effect, weighted sensitivity and specificity with 95% confidence intervals were calculated using data corrected for the clustering of prolapsing segments within patients. The subjects with nondiagnostic image quality were excluded from segment analysis. In addition to the segmental-related analysis, we analyzed the 2D and RT3D echocardiographic accuracy for patient-related MV prolapse localization. The inability of echocardiographic imaging to recognize and/or assign prolapse lesions to the surgical localization was calculated as a false result. For the purpose of the statistical analysis, the multiscallops P2, observed between P1 and P3, were classified as P2. Receiver operating characteristic curve analysis was used to select the best cutoff value of segmental MV displacement measured with RT3D-TEE for prolapse diagnosis. The inter- and intraobserver agreement in the echocardiographic images was assessed using the κ test. Agreement or disagreement with the surgical findings and different significance in diagnostic accuracy between the echocardiographic images were assessed using the McNamara test. p Values <0.05 were considered statistically significant. The statistical analysis was performed using the Statistical Package for Social Sciences software, version 15 (SPSS, Chicago, Illinois).
Results
Interpretable RT3D-TEE was feasible in all patients, with optimal (90%) and suboptimal (10%) imaging, although off-line reconstruction, removing artifacts, and/or optimizing the ultrasound gain and the smoothing setting improved the suboptimal quality of the examinations. All RT3D-transesophageal echocardiographic zoom mode studies were suitable for quantitative analysis. However, the full-volume data sets were excluded because of the high incidence (60%) of stitching artifacts during off-line cropping. RT3D-TTE was interpretable in 149 patients, but diagnostically inadequate in 73 of the 222 patients. Owing to the high rate of noninterpretable or suboptimal examination, we also excluded RT3D-TTE for quantitative off-line analysis. Figure 2 shows localization of the MV prolapse, according to the surgical findings for the 222 patients. Of the prolapsed segments, 263 were classified as dominant by the surgeon and 25 as secondary. The per-patient analysis showed a monoleaflet localization of the prolapsed segments in 181 of 222 patients, involving 1 segment in 162 (17 anterior leaflets and 145 posterior leaflets) and ≥1 segment in 19 patients (9 anterior leaflets and 10 posterior leaflets). Thirty-three patients showed a bileaflet prolapse with facing or nonfacing localization in 31 and 2 patients, respectively. In the remaining 8 patients, the prolapse was confined to the medial commissure (2 patients) or combining commissure and leaflet localization (6 patients). Rupture of chordae tendinee was observed in 127 patients (57%), involving the posterior leaflet in 111, anterior leaflet in 15, and posteromedial commissure in 1 patient. The cause of MV disease was myxomatous degeneration in 187 patients (84%), fibroelastic deficiency in 13 (6%), and indeterminate in 22 (10%). Myxomatous degeneration was diffuse in 96 patients but was limited to the prolapsed segment in 91 patients.
Of 222 patients, 202 (91%) showed successful repair (absence or mild 1+ residual mitral regurgitation), confirming the appropriate repair-targeting surgical diagnosis. In contrast, unsuccessful first-pump-running MV repair was found in 20 of 222 patients with residual ≥2+ mitral regurgitation related to unrecognized secondary prolapse (12 patients), persistent systolic anterior motion after optimization of the load condition (2 patients) or complex prolapse lesions associated with annular calcification (6 patients). A second pump run was performed to optimize the repair strategy (14 patients) or replace the mitral valve (6 patients with annular calcification). Twelve patients were discharged with less-than-perfect repair results (residual mild regurgitation) at 2D-TTE (8 patients with posterior leaflet prolapse, 2 with bileaflet prolapse, and 2 with combined prolapse).
The per-segment accuracy values of RT3D-TEE compared to 2D-TEE, RT3D-TTE, and 2D-TTE for localization of surgically MV prolapsed lesions are listed in Table 2 . Taking into account the clustering of prolapsing segments within patients, the weighted sensitivity and specificity for RT3D-TEE were significantly greater than those of alternative echocardiographic modalities. Receiver operating characteristic curve analysis identified the ≥5-mm tip leaflet displacement, assessed using multiplanar reconstruction, as the best cutoff value for surgically diagnosed prolapse. Figure 3 shows that ≥5-mm leaflet displacement significantly improved the positive predictive value for prolapsed segment diagnosis using RT3D-TEE compared to the ≥2 mm cutoff. We also used the 2 different multiplanar reconstruction cutoffs (≥5 mm and ≥2 mm to <5 mm) to distinguish between dominant and secondary prolapsed lesions. Using these multiplanar reconstruction criteria, RT3D-TEE identified all surgically dominant and secondary prolapsed segments. However, 48 of the 73 echocardiographically defined secondary lesions were not surgically confirmed, leading to low positive predictive and high negative predictive values for the secondary prolapse diagnosis (34% and 100%, respectively; Table 3 ).
| Echocardiographic Findings | Surgical Findings | Total Segments (n = 1,776) | |
|---|---|---|---|
| Prolapse (n = 288) | Normal (n = 1,488) | ||
| Two-dimensional transthoracic echocardiography ⁎ | |||
| Prolapse | 213 | 120 | 333 |
| Normal | 75 | 1,264 | 1,339 |
| Nondiagnostic | 104 | ||
| RT3D-TTE † | |||
| Prolapse | 176 | 37 | 213 |
| Normal | 20 | 959 | 979 |
| Nondiagnostic | 584 | ||
| 2D-TEE ‡ | |||
| Prolapse | 272 | 120 | 392 |
| Normal | 16 | 1,368 | 1,384 |
| Nondiagnostic | 0 | ||
| RT3D-TEE § | |||
| Prolapse | 288 | 48 | 336 |
| Normal | 0 | 1,440 | 1,440 |
| Nondiagnostic | 0 | ||
⁎ W average sensitivity 0.56 (95% CI 0.46–0.65), W average specificity 0.90 (95% CI 0.89–0.91).
† W average sensitivity 0.88 (95% CI 0.81–0.96), W average specificity 0.96 (95% CI 0.95–0.97).
‡ W average sensitivity 0.95 (95% CI 0.89–1.01), W average specificity 0.92 (95% CI 0.91–0.94).
§ W average sensitivity 1 (95% CI 0.94–1.06), W average specificity 0.97 (95% CI 0.96–0.97).
| RT3D-TEE | Surgical Findings | |||
|---|---|---|---|---|
| Prolapse (n = 288) | Normal (n = 1,488) | Total segments (n = 1,776) | ||
| Dominant (n = 263) | Secondary (n = 25) | |||
| Prolapse | ||||
| Dominant (≥5 mm) | 263 | 0 | 0 | 263 |
| Secondary (≥2 to <5 mm) | 0 | 25 | 48 | 73 |
| Normal | 0 | 0 | 1,440 | 1,440 |
The agreement between the echocardiographic and surgical findings according to the per-patient MV mapping of the prolapsed site is listed in Table 4 . Prolapse localization, including both dominant and secondary lesions, was correctly identified by RT3D-TEE in 204 (92%) of 222 patients compared to 2D-TEE, RT3D-TTE, and 2D-TTE, which identified 173 (78%) of 222 (p <0.001), 119 (80%) of 149 (p <0.001), and 114 (54%) of 209 (p <0.0001) patients, respectively. Although RT3D-TTE showed high diagnostic accuracy in the context of the interpretable examination, the overall accuracy was lower (54%) because of the high percentage of nondiagnostic images in 73 of the 222 patients. Even among those 60 patients with complex prolapse (>1 segment localization or commissural lesions), RT3D-TEE correctly identified 58 (96.5%) compared to 42 (70%), 31 (52%), and 21 (35%) detected using 2D-TEE, RT3D-TTE, and 2D-TTE (p <0.0001), respectively. In 18 (8%) of 222 patients, RT3D-TEE showed incomplete agreement with the surgical diagnosis owing to the secondary lesions that were not recognized by anatomic observation. Therefore, using multiplanar reconstruction with ≥5-mm and ≥2 to <5-mm displacement as the respective threshold values for RT3D-TEE for dominant and secondary prolapse, we achieved complete agreement between the echocardiographic and surgical findings for the recognition of dominant prolapse (accuracy 100%) but incomplete agreement for the diagnosis of both dominant and secondary prolapse (accuracy 92%). Figure 4 shows the overall rate of surgical agreement with echocardiographic findings in prolapse classification in patients with simple-monoleaflet disease compared to complex disease.



