Our study aimed to elucidate mechanisms underlying discordance between fractional flow reserve (FFR) and hyperemic stenosis resistance (hSR) in some patient subsets. To do this, we enrolled 30 consecutive patients with stable angina or non–ST elevation myocardial infarction (non-STEMI) and with a nonculprit intermediate coronary lesion (40% to 70%) by coronary angiography. We measured aortic pressure, flow velocity, and pressure distal to lesion simultaneously at basal level and during adenosine-induced (fixed intracoronary dose of 120 μg) hyperemia using a dual-sensor–equipped guidewire. Microvascular resistance (MR; pressure distal to lesion/flow velocity, mm Hg/cm/s) and variation (Δ) in MR levels were calculated both at baseline and after hyperemia, whereas FFR (cutoff <0.80) and hSR [(aortic pressure − pressure distal to lesion)/flow velocity, cutoff >0.80 mm Hg/cm/s] were assessed after intracoronary adenosine. Twenty-three patients (76.7%) showed concordance and 7 patients (23.3%) showed discordance between FFR and hSR (all cases with FFR >0.80 and hSR >0.80). Discordant patients presented more frequently with non-STEMI (85.7% vs 39.1%, p = 0.04), significantly higher C-reactive protein serum levels (median [interquartile range] 5.9 [5.1 to 6.8] vs 4.9 [3.7 to 6.2] mg/L, p = 0.007), and lower ΔMR (p = 0.03) values compared with concordant patients. In conclusion, patients with non-STEMI and those with increased C-reactive protein levels show a lower reduction in MR after intracoronary adenosine–induced hyperemia, leading to FFR underestimation.
The presence of myocardial ischemia is an important risk factor for adverse clinical outcome. When revascularization is limited to stenotic coronary lesions that induce ischemia, functional status and outcome improve. Fractional flow reserve (FFR) is a physiological parameter based on measurement of intracoronary pressure, provides a framework for assessing the hemodynamic significance of coronary stenoses, and has been proven by multiple randomized studies to be a reliable instrument for guiding clinical decision making and improving treatment appropriateness and outcomes. As with any clinical technique, the ease of use that facilitated its development also led to several theoretical limitations and potential pitfalls. One of these is FFR reliance on attainment of pharmacologic maximal hyperemia, which may not be accomplished in situations in which maximal lowering of microvascular resistance (MR) could not be achieved, such as acute coronary syndromes, microvascular diseases, and situations characterized by increased inflammatory biomarker serum levels. Indeed, all these clinical situations usually leave the microcirculation prone to vasodilatory impairment. Of note, FFR may be also overestimated in clinical situations in which baseline coronary flow could be increased. Hyperemic stenosis resistance (hSR) is a combined index of pressure and flow, expressed in mm Hg/cm/s, proven to be a more powerful predictor of reversible perfusion defects in a population of patients with non–ST elevation myocardial infarction (non-STEMI) and stable angina. In this study, we aimed at elucidating mechanisms underlying the discordance between FFR and hSR in some patient subsets.
Methods
We enrolled 30 consecutive patients undergoing coronary angiography and having an intermediate nonculprit coronary stenosis (40% to 70%) from November 2012 to February 2013; 15 patients presented with stable angina and 15 patients with non-STEMI. Non-STEMI was defined as chest pain at rest in the last 48 hours preceding the admission associated with evidence of transient ST-segment depression on 12-lead electrocardiogram and elevated troponin-T serum levels. Stable angina was defined as angina on effort with a stable pattern of symptoms for at least the last 6 months before admission. All patients were enrolled after performing coronary angiography showing a culprit coronary lesion and an intermediate lesion in another vessel. Exclusion criteria were significant stenosis of the left main coronary artery (n = 2 patients), recent myocardial infarction (n = 3 patients), previous cardiac surgery (n = 4 patients), hypertrophic cardiomyopathy (n = 1 patient), ejection fraction <30% (n = 3 patients), severe valvular abnormalities (n = 2 patients), coagulation abnormalities (n = 1 patient), severe renal insufficiency (n = 2 patients), severe chronic heart failure (New York Heart Association class III to IV, n = 3 patients), inflammatory diseases or acute and chronic infections (n = 1 patients), recent surgery or trauma (within 3 months, n = 1 patients), neoplastic processes potentially responsible for systemic activation of the inflammatory process (n = 1 patient), or use of anti-inflammatory drugs and/or immunosuppressants (n = 1 patients). Of note, 16 patients presenting with an intermediate coronary stenosis and eligible for the study denied informed consent. All patients enrolled in the study underwent physiological assessment of coronary stenosis, as described in the following, at the same time of culprit lesion treatment, along with measurement of systemic C-reactive protein (CRP) serum levels. Of note, physiological assessment of coronary stenosis was performed within 72 hours from the onset of chest pain for patients with non-STEMI. FFR and hSR were evaluated and thresholds were FFR <0.80 and hSR >0.80. Patients were classified as concordant or discordant based on the agreement of these 2 indexes: concordant patients were defined as having FFR <0.80 and hSR >0.80 or FFR ≥0.80 and hSR ≤0.80 and discordant patients as having FFR <0.80 and hSR ≤0.80 or FFR ≥0.80 and hSR >0.80.
In all patients, cardiovascular risk factors were carefully examined, including family history of early coronary artery disease (first-degree relative with a history of myocardial infarction <60 years), diabetes (fasting blood glucose >126 mg/dl or treated diabetes), dyslipidemia (low-density lipoprotein >130 mg/dl, high-density lipoprotein <45 mg/dl, triglycerides >150 mg/dl, or total cholesterol >200 mg/dl) smoking, and hypertension (systolic blood pressure >140 mm Hg and/or diastolic blood pressure >90 mm Hg or treated hypertension), and therapy administered at the time of cardiac catheterization was recorded. The study was approved by the local ethics committee, and after a complete explanation of the aims and details of the study, all patients gave their informed consent before entering the study.
Blood samples were drawn, before coronary angiography, from a brachial vein in all patients and collected in ethylenediaminetetraacetic acid tubes or tubes without any anticoagulant and centrifuged. Plasma and serum aliquots were stored at −80°C in appropriate cuvettes until assayed. Troponin T was measured using Roche Diagnostics reagents on an Elecsys 2010 immunoassay analyzer (F. Hoffmann-La Roche Ltd, Basel, Switzerland). The criterion of coefficient of variation ≤10% in our laboratory was met by a troponin-T concentration of ≥0.03 ng/ml. CRP was also measured by an ultrasensitive nephelometric method (Dade Behring Latex BN2, Marburg, Germany, lower detection limit: 0.175 mg/L).
The angiographic analysis (quantitative coronary angiography) was conducted in a blinded fashion by 2 experienced cardiologists (GN and NC), unaware of the identity and clinical and laboratory characteristics of the patients enrolled in the study. The angiographic images were acquired at 15 frames/s. The 2-dimensional quantitative analysis was performed offline using the CAAS II system (Pie Medical Imaging, Maastricht, Netherlands). An automatic calibration was obtained using the guiding catheter as reference. All analyses were conducted during diastole, frame focusing on projections in which the prospective shortening of the stenosis was minimal.
The functional analysis of intermediate stenosis was conducted using pressure flow–combined guidewires (ComboWire; Volcano Corporation, San Diego, California). Basal and hyperemic values of aortic pressure (mm Hg), pressure distal to lesion (mm Hg), and blood flow velocity (cm/s) were recorded. Hyperemia was obtained by an intracoronary bolus of 120 μg of adenosine.
FFR (hyperemic pressure distal to lesion/hyperemic aortic pressure), coronary flow reserve (hyperemic blood flow velocity/basal blood flow velocity), hSR [(hyperemic aortic pressure − hyperemic pressure distal to lesion)/hyperemic blood flow velocity, mm Hg/cm/s], MR index at hyperemia (hMR = hyperemic pressure distal to lesion/hyperemic blood flow velocity, mm Hg/cm/s) and at baseline (basal pressure distal to lesion/basal blood flow velocity, mm Hg/cm/s), and MR gradient (ΔMR = MR index at baseline − hMR, mm Hg/cm/s) were consequently calculated.
Continuous variables are expressed as mean ± SD or median (interquartile range) if they followed a normal or non-normal distribution, respectively, and dichotomous variables as percentages. Unpaired t test or Mann-Whitney U test was used for comparison between the 2 groups; categorical variables were compared using the chi-square test or Fisher’s exact test, as appropriate. Correlations between continuous variables were analyzed using Pearson or Spearman test, as appropriate. Statistical significance was considered for values of p <0.05. All analyses were performed with SPSS 17.0 (SPSS Science, Chicago, Illinois).
Results
Table 1 lists main clinical, angiographic, and laboratory data of all patients enrolled in our study. Overall, 30 patients were enrolled (15 patients with stable angina [age 69 ± 8 years, male gender 73%] and 15 patients with non-STEMI [age 66 ± 9 years, male gender 73%]). The 2 groups were similar with regard to the main clinical and angiographic characteristics and therapy before coronary angiography, except for CRP serum levels which were higher in patients presenting with non-STEMI compared with those presenting with stable angina (5.20 [4.90 to 6.35] vs 2.30 [1.90 to 3.00] mg/L, p <0.001).
| Patient | Age (yrs) | Sex | Arterial Hypertension | Current Smoker | Dyslipidemia | Family History of Coronary Disease | Diabetes Mellitus | β Blockers ∗ | ACE Inhibitors ∗ | Statins ∗ | Aspirin ∗ | Left Anterior Descending (Culprit Vessel) | Multivessel Disease (≥2) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stable angina | |||||||||||||
| 1 | 54 | F | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes | No | No |
| 2 | 59 | M | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes |
| 3 | 59 | F | Yes | Yes | No | No | No | Yes | Yes | No | Yes | Yes | Yes |
| 4 | 61 | M | No | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes |
| 5 | 62 | M | Yes | No | Yes | No | No | Yes | Yes | Yes | Yes | Yes | No |
| 6 | 68 | F | Yes | No | Yes | No | Yes | Yes | Yes | Yes | Yes | No | No |
| 7 | 70 | M | Yes | No | No | Yes | No | Yes | Yes | No | Yes | Yes | Yes |
| 8 | 71 | M | Yes | No | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 9 | 71 | M | No | No | Yes | No | Yes | No | No | Yes | Yes | Yes | Yes |
| 10 | 72 | M | Yes | No | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes |
| 11 | 74 | M | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | No |
| 12 | 74 | M | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes |
| 13 | 77 | F | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes |
| 14 | 79 | M | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | No | No |
| 15 | 83 | M | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes |
| Unstable angina | |||||||||||||
| 1 | 52 | M | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | No | No |
| 2 | 53 | M | No | Yes | Yes | No | No | No | No | Yes | Yes | No | No |
| 3 | 56 | M | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes |
| 4 | 58 | M | No | No | Yes | No | No | Yes | Yes | Yes | Yes | Yes | No |
| 5 | 61 | M | Yes | No | No | No | No | Yes | Yes | Yes | Yes | Yes | Yes |
| 6 | 64 | F | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No |
| 7 | 66 | F | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | No |
| 8 | 68 | M | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 9 | 69 | M | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes |
| 10 | 69 | M | No | No | Yes | No | No | Yes | Yes | Yes | Yes | Yes | No |
| 11 | 70 | M | No | Yes | No | Yes | No | Yes | Yes | Yes | Yes | No | Yes |
| 12 | 72 | M | Yes | No | Yes | No | No | Yes | Yes | Yes | Yes | No | No |
| 13 | 78 | F | Yes | Yes | No | No | No | Yes | Yes | No | Yes | Yes | Yes |
| 14 | 79 | M | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 15 | 82 | F | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No |
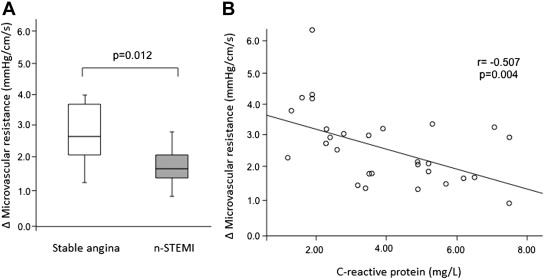
Patients presenting with non-STEMI had higher hMR values along with lower ΔMR and coronary flow reserve values compared with patients presenting with stable angina (2.35 [2.05 to 3.30] vs 1.93 [1.63 to 2.48] mm Hg/cm/s, p = 0.027; 1.64 [1.41 to 2.69] vs 2.75 [2.06 to 3.93] mm Hg/cm/s, p = 0.012; and 1.48 [1.35 to 1.62] vs 2.00 [1.69 to 2.56] mm Hg/cm/s, p = 0.001; Figure 1 ).