Imaging for Endovascular Therapy
Hugh G. Beebe
Before the widespread application of endovascular treatment for common vascular conditions, the interest of most vascular surgeons in imaging was quite limited, in terms of both imaging techniques and the information derived from imaging. Before the endovascular era, images were primarily used to establish indications for surgical treatment, aneurysm size, or degree of internal carotid artery stenosis, but beyond that, information from images was used only in a general and qualitative way for planning operations. Direct surgical exposure allowed the surgeon control of anatomy, and experienced operators could readily make decisions by observation. But everything changed when surgeons began to include endovascular techniques into their practice as a central part of managing all vascular disease. Endovascular treatment requires precise definition of the extent of disease and accurate measurement of the dimensions of a vascular segment that is to be structurally altered by endovascular devices. The endovascular surgeon must have a thorough understanding of the artifacts and errors that are part of all imaging methods. Modern vascular surgeons should have practical working skills in the following areas:
Imaging for patient selection for intervention
Device selection for treatment
Imaging for procedure guidance
These large fields of knowledge contain many subjects and are certainly too large to be discussed completely here. This chapter seeks to provide an approach to using imaging as the centerpiece of current vascular surgery practice by reviewing generally familiar vascular imaging methods with emphasis on the importance of major artifacts, errors, and limitations. This chapter also summarizes the growing trend toward routine three-dimensional (3-D) image processing and explains why this technique is important to vascular surgeons.
Arteriography
Contrast arteriography is the most familiar form of vascular imaging that is still widely used despite attempts to displace it with other methods that do not have the liabilities of cost, radiation and contrast toxicity, and image storage and retrieval difficulties. It is most useful for procedure guidance at the time of performing a catheter-based intervention, such as angioplasty or stent graft insertion. When surgical bypass was the primary treatment option for lower extremity arterial occlusive disease, arteriography was used extensively for planning the operation, but even then it tended to be used only in a qualitative sense. The usual mindset of the surgeon was, “We’ll do a femorotibial bypass to the midposterior tibial artery; we’ll see how it looks when we get there.” There was no need to know the true diameter of the vessels or the length of the arteriosclerotic lesion causing occlusion, because the sites of anastomosis could be seen and the lesion was being bypassed. However, planning balloon angioplasty with or without stenting requires selection of a balloon of appropriate size (in both diameter and length), and among a wide array of devices that are used to treat lower extremity arterial occlusive disease, patient selection and choice of device are driven by image-based measurements.
Even though arteriography has stood the test of time as a useful and classical imaging method, it can deceive in many ways if taken at face value. Understanding the many errors and artifacts of arteriography helps to make the method more useful. The lower extremity arteriogram reveals arterial occlusion by what it does not show rather than by what it does show. And since arterial thrombosis extends over a vessel length until collateral flow acts to limit it, nonvisualizing artery is usually longer, often much longer, than the actual length of the arteriosclerotic lesion that caused the thrombosis.
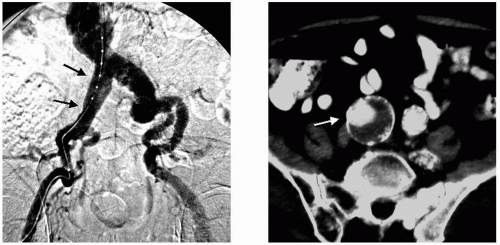
Another important problem with arteriograms is the individually variable magnification artifact. The x-ray beams traveling through the patient diverge in a cone shape from the tube to the film or image recorder. Thus the object-to-film distance, the aorta lying in the posterior abdomen, for example, will vary according to body habitus. A man weighing 275 pounds will have a larger magnification error of his aortogram than a 90-pound woman, though both of them may have a 5-cm abdominal aortic aneurysm (AAA) diameter. The range of magnification artifact commonly seen in clinical practice can vary from 15% to as much as 35%. The use of marker catheters for arteriography helps to overcome this problem, but careful measurement is still needed to compensate for catheter position that is not perpendicular to the x-ray beam (Fig. 3-1).
Thrombus artifact is another shortcoming of arteriography, because the arteriogram shows the lumen and not the noncalcified blood vessel wall. Thus a symmetrical thrombus within an aneurysm that narrows the flow lumen greatly or to the size of the normal arterial segment commonly prevents an arteriogram from revealing a significant aneurysm’s true size or its presence at all (Fig. 3-2).
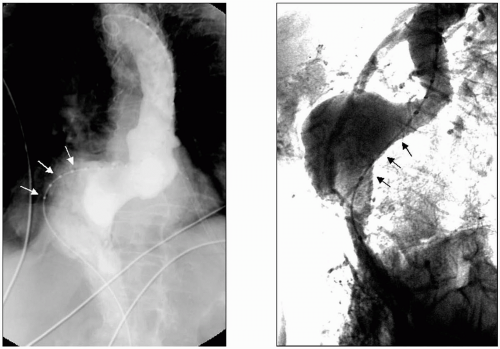
It is common practice for clinical decisions to be made based on single-projection arteriograms. But in the case of the abdominal aorta or the carotid bifurcation, among many other examples, an arteriogram obtained from different projection angles reveals huge differences in angulation or in the apparent arterial stenosis. This effect of projection angle is especially critical when using fluoroscopy and arteriography to guide aortic stent graft insertion. If the infrarenal aortic attachment zone (“aortic neck”) is greatly angled in an anterior-posterior plane, as is often the case in large aneurysms, failure to appreciate this leads to a failure to angle the x-ray C-arm so that the true length of the aortic neck is shown. This simple imaging error has been the cause of unnecessarily misplaced aortic stent grafts (Fig. 3-3).
Another aspect of fluoroscopy and arteriography that needs more consideration than it often gets is a conscious effort to learn how to limit the amount of fluoroscopy time and contrast use, especially during aortic aneurysm stent graft exclusion. It should become a habit of the skilled endovascular therapist to incorporate a minimalist approach to procedure guidance x-ray imaging and not only for the obvious reasons of limiting radiation exposure and contrast load. Another reason that arises occasionally is the development of an unanticipated complex problem during endovascular aortic aneurysm treatment, such as type I endoleak, which doesn’t occur until late in the procedure and may require extended imaging to solve.
Computerized Tomography
Although many radiology and surgery departments share well-founded enthusiasm for magnetic resonance imaging (MRI), there is general consensus favoring computerized tomography (CT) for an increasing number of vascular imaging applications. CT requires radiation exposure and the use of significant amounts of contrast agent, but the use of sophisticated post processing allows most vascular conditions to be completely displayed and measured through a single CT acquisition with nearly complete freedom from artifacts. The advent of multidetector CT scanning equipment so improves quality and resolution of images obtained in short intervals of time that it offers a qualitative leap forward in anatomic fidelity and accuracy of post processing methods. In recent years the number of detectors of the new generation of CT scanners has multiplied from a single detector to as many as 64. This acquires many slices (up to 64) in a single rotation and at a slice thickness of a millimeter or even less. The effect is greater accuracy because of virtually eliminating motion artifact and achieving very thin tissue volumes in the image data. All radiographic images depend on radiodensity differences to produce a picture that can be anatomically interpreted. If the tissue volumes of the image acquisition are relatively large, the volume averaging effect within each small dataset that makes up the total image will blur the differences more than would happen with a small tissue volume.
The use of 2-D axial CT images is heavily burdened with artifacts and inaccuracies. Among them is the artifactual elliptical




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree