Chapter 16 Iliofemoral Venous Occlusive Disease
Historical Background
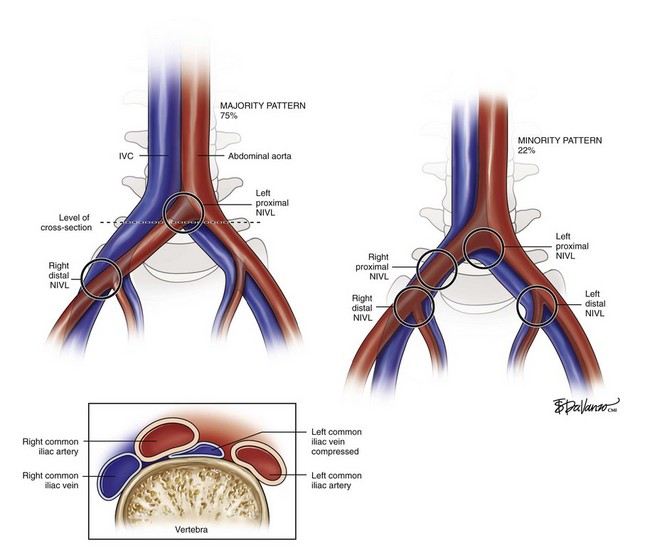
In approximately 40% of patients, the precursor to iliac vein obstruction is not thrombosis; rather, a nonthrombotic iliac vein lesion (NIVL), also known as May-Thurner syndrome1 or iliac vein compression syndrome,2 causes obstruction. A nonthrombotic blockage is defined as an absent clinical history of deep venous thrombosis coupled with absent findings on imaging studies such as contrast venography and ultrasound. Typically, an NIVL is caused by a stenosis of the left proximal common iliac vein by the right common iliac artery with secondary band or web formation.3 This lesion is classically found in the left common iliac vein of younger females, but it is not uncommon in males or in elderly patients and it may involve the right limb. At least 15% of patients with limbs with primary disease have been shown to have stenosis of both common and external iliac veins.4
As depicted in Figure 16-1, the right common iliac artery always crosses the left common iliac vein at the confluence of the IVC and is where classic proximal left NIVL occurs. In 75% of cases (majority pattern), the right iliac artery continues its course to cross near the external iliac vein level, in which case it may induce right distal NIVL. In the minority pattern (22%), the right common iliac artery crosses the right common iliac vein; it then courses down over a longer length of the external iliac vein; it can thus induce either a proximal or distal right NIVL. The left distal lesion may be related to the crossing of the vein by the left hypogastric artery. These anatomic variations may explain why proximal NIVLs occur more frequently on the left side than on the right side, why the left lesion is focal and the right is less so, and why the distal NIVL occurs equally on either side.
Although traditional venous corrective surgery concentrated on the correction of venous reflux below the inguinal ligament, the introduction of minimally invasive venous stenting with venography and intravenous ultrasonography (IVUS) provides the ability to treat the “obstructive” component of the disease above the inguinal ligament. The emphasis on IVUS as the key diagnostic tool was promulgated by Raju et al.,5 who have shown that iliac venous stenting alone is sufficient to control symptoms in most patients with combined outflow obstruction and deep reflux.
Etiology and Natural History of Disease
The iliac vein is the common outflow tract of the lower extremity, and chronic obstruction of this segment can result in severe symptoms. The most frequent precursor to chronic pelvic venous outflow obstruction is iliofemoral deep venous thrombosis. Only 20% to 30% of iliac veins completely recanalize spontaneously after thrombosis, whereas the remaining veins recanalize partially and develop varying degrees of collaterals.6 This may result in significant remaining obstruction to the venous outflow of the lower extremity. In the iliofemoral segment, collateral vein formation is relatively poor and thus results in more severe symptoms than does lower segmental blockage.7 Distal obstructions are more readily compensated for because of robust femoropopliteal collaterals, profunda–popliteal vein connections, deep muscular tributaries in the thigh, and saphenosaphenous venous connections.
The high prevalence and pathologic role of NIVLs in patients with symptoms of chronic venous disease must be balanced with the frequent prevalence of NIVLs in the asymptomatic general population. These apparent contradictions can be resolved if the NIVL is viewed as a permissive condition predisposing to the development of chronic venous disease. Permissive conditions are diseases that may remain silent until additional insult or abnormality is superimposed. Symptom expression in many NIVL obstructions remains asymptomatic because it probably is a slowly progressive condition. Additional insult or pathologic conditions, such as trauma, cellulitis, distal thrombosis, secondary lymphatic exhaustion, or reflux, may render the extremity symptomatic.8
In Raj and Neglen’s series,8 75% of limbs with NIVLs and concurrent reflux experienced a good or excellent outcome with stent placement alone, even when the reflux component, severe in many, was uncorrected. These results support the concept that the NIVL plays a permissive role in the genesis of chronic venous disease symptoms. NIVLs are ubiquitous in chronic venous disease with severe symptoms, and liberal use of IVUS and stent correction when they are detected is recommended. Even patients with significant distal reflux are not precluded if the stents fail later, thus avoiding or postponing open reflux corrective procedures.
When symptoms recur after initial remission following stent placement, stent malfunction is often found. Distal migration of the stent with recurrence of the lesion at the iliocaval junction, missed or incomplete treatment of the distal lesion, or, less commonly, in-stent stenoses were found in Raju and Neglen’s series.8 When corrected, the patient is usually returned to the status of symptom remission.
Patient Selection
When planning an invasive procedure on a patient, a surgeon preferably enters the operating room with an imaging study demonstrating a pathologic lesion. In the case of iliac vein occlusive disease, this is often not possible. The poor diagnostic sensitivity of venography was well documented by Negus et al.3; of practical importance is that up to half of the cases can be missed if frontal projection venograms alone are relied on for diagnosis.
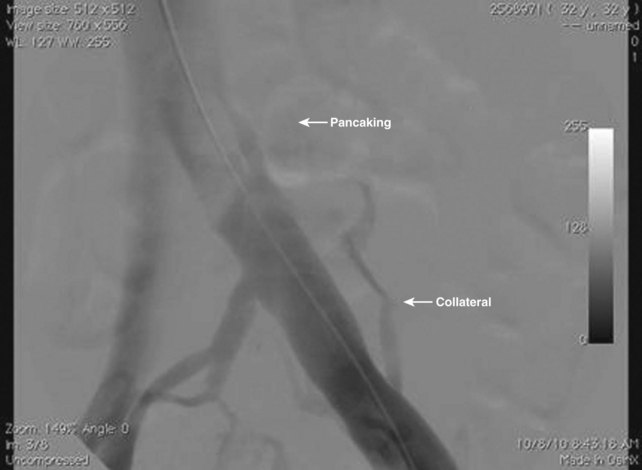
Perhaps one of the major reasons why interest in iliac vein corrective surgery has lagged is the discomfort a surgeon feels when entering the operating room without a definitive diagnosis. Raju and Neglen8 have one of the largest experiences in the world with iliac vein occlusive disease, and they have emphasized the value of intraoperative IVUS for diagnosis and treatment. Their vascular laboratory runs a battery of noninvasive tests on patients with signs and symptoms of venous disease, and their data demonstrate the insensitivity of these examinations to identify lesions8 (Table 16-1). Magnetic resonance venography is operator dependent, and its routine use has not elevated this technique as the gold standard for diagnosis. Perhaps the most interesting phenomenon is the insensitivity of intravenous contrast venography. Although anteroposterior and oblique views may suggest some “pancaking” of the proximal iliac vein (Fig. 16-2), the common iliac vein often appears normal.
![]() TABLE 16–1 Demographics, Intravascular Ultrasound Findings, and Preinterventional Hemodynamics in Stented Limbs with Nonthrombotic Iliac Vein Lesions with and Without Venous Reflux
TABLE 16–1 Demographics, Intravascular Ultrasound Findings, and Preinterventional Hemodynamics in Stented Limbs with Nonthrombotic Iliac Vein Lesions with and Without Venous Reflux
| Parameter | NIVLs With Reflux (n = 151) | NIVLs Without Reflux (n = 181) |
|---|---|---|
| Age, y | 56 (20-85) | 51 (18.90)* |
| Female-male ratio | 110 : 36 (3.1 : 1) | 146 : 31 (4.7 : 1) |
| Left-right limb ratio | 105 : 46 (2.3 : 1) | 136/45 (3 : 1) |
| IVUS degree of stenosis, % | 70 (0-95) | 70 (0-100) |
| Stenotic area, cm2 | 0.66 (0.15-2.00) | 0.53 (0.02-1.65)* |
| Ambulatory venous pressure, % drop | 77 (0-97) | 77 (0-99) |
| Venous filling time, sec | 23 (2-132) | 44 (0-165) |
| APG:VFI90, mL/sec | 2 (0-12.3) | 0.9 (0.0-6.0) |
| Hand-foot pressure differential, mm Hg | 1 (0-8) | 1 (0-10) |
| Dorsal foot hyperemia pressure differential, mm Hg | 6 (0-26) | 5 (0-23) |
Data are presented as ratio or median (range).
*p < .01, Not significant.
APG, Air plethysmograph; NIVI, Nonthrombotic iliac vein lesions; IVUS, intravascular ultrasound; VFI90, venous filling index.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree