Chapter 11 Treatment of Spider Telangiectasias
Historical Background
Although the treatment of varicose veins was in the phase of further refinement, the treatment of telangiectasias was not seriously attempted until the 1930s. It was Biegeleisen who is credited with initially attempting injection into the perivascular space around telangiectatic areas. Later, he implemented intravascular injections using homemade microneedles.1 These early efforts led to disappointing results, primarily because the sclerosing solutions, such as sodium morrhuate, were very caustic. It was not until the 1970s that others attempted to treat spider telangiectasias with intravascular injection using less caustic solutions such as sodium tetradecyl sulfate (STS) (Sotradecol) and hypertonic saline. It was these agents that propelled the treatment of telangiectasias forward. The enthusiasm for these treatments increased steadily as Foley’s publication, relating to this new technique, gained momentum.2
Etiology
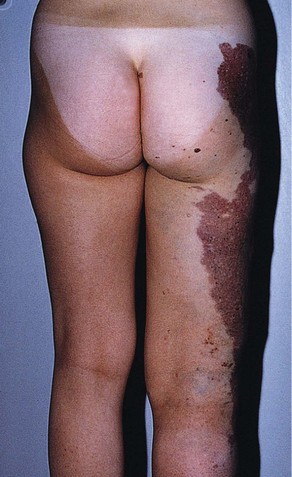
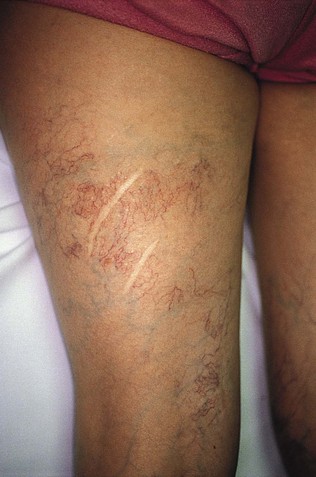
Although research continues to be done in this area, there is consensus today that telangiectasias result from a number of causes, alone or more likely in combination with other etiologic factors. Telangiectatic leg veins, according to the contemporary research, arise as a result of venous hypertension secondary to a number of different causes and conditions. The etiology of varicose veins and telangiectasias, for the most part, is similar. The pathophysiology of telangiectasias is usually broadly categorized as genetic/congenital, acquired and iatrogenic. Some of the genetic causes of telangiectasias include nevus flammeus (port- wine stains), nevus araneus (spider telangiectasia, which can also result from acquired diseases), and Klippel-Trenaunay syndrome. Congenital conditions associated with telangiectasias include Maffucci syndrome and Rothmund-Thomson syndrome (poikiloderma). Acquired causes of telangiectasias can arise from a primary cutaneous disorder, such as varicose veins and keratosis lichenoides chronica, or the result of a disorder with a secondary cutaneous component, such as lupus erythematosus, a collagen disorder, and mastocytosis (telangiectasia macularis eruptiva perstans). Hormonal influences (estrogen and progesterone) also play a role in the pathogenesis of telangiectasia. Pregnancy places the person at risk for the development of telangiectasia as early as a couple of weeks after conception. Birth control pills, menses, and the time just before ovulation are also associated with the development or worsening of telangiectasia, and increased venous dispensability. Topical steroids, particularly at high doses, have also been identified as a possible causative factor. Last, physical insults, like trauma (contusions) and infection, have also been implicated as causal forces. See Box 11-1 for a comprehensive listing of the many causes of lower leg cutaneous telangiectasia.
Box 11–1 Causes of Cutaneous Telangiectasia of the Lower Extremities
Congenital Neuroangiopathies
Congenital poikiloderma (Rothmund–Thomson syndrome)
Essential progressive telangiectasia
Cutis marmorata telangiectatica congenita
Diffuse neonatal hemangiomatosis
Other Acquired/Primary Cutaneous Diseases
Necrobiosis lipoidica diabeticorum
Capillaritis (purpura annularis telangiectodes)
Infection
Generalized essential telangiectasia
Progressive ascending telangiectasia
Human immunodeficiency virus (HTLV-III)
Modified from Goldman MP, Bennett RG. J Am Acad Dermatol 1987;17:167.
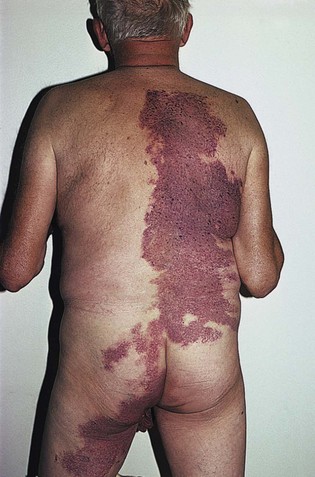
Fig 11–4 Nevus flammeus.
(From Weiss RA, Goldman MP, Bergan JJ, et al. Sclerotherapy: Treatment of Varicose and Telangiectatic Leg Veins. St Louis: Elsevier, 2007, Fig. 4.6, p. 76.)
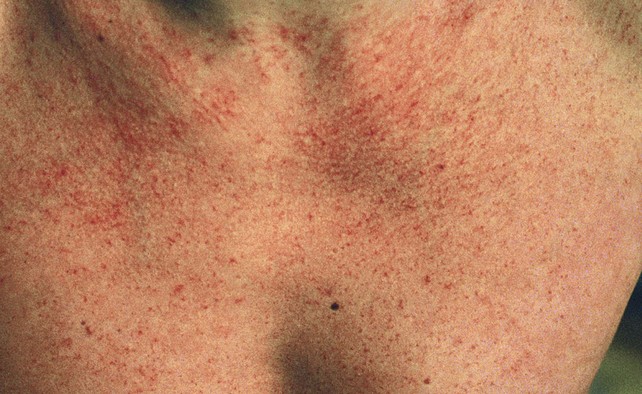
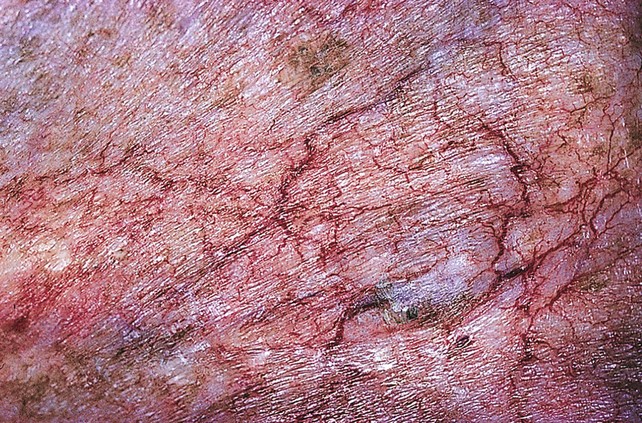
Fig 11–6 Extensive fine red telangiectasia on the chest of a severely sun-damaged 50-year-old woman.
(From Weiss RA, Goldman MP, Bergan JJ, et al. Sclerotherapy: Treatment of Varicose and Telangiectatic Leg Veins. St Louis: Elsevier, 2007, Fig. 4.19, p. 85.)
Endovascular Instrumentation
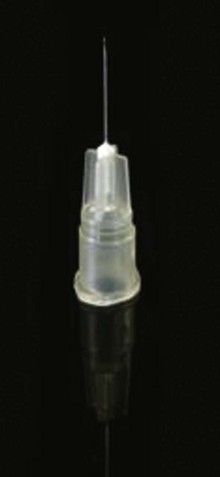
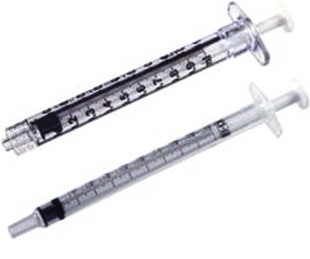
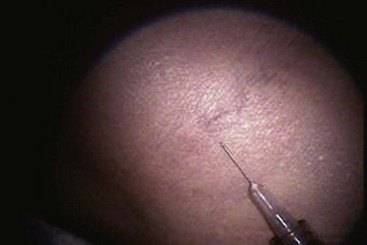
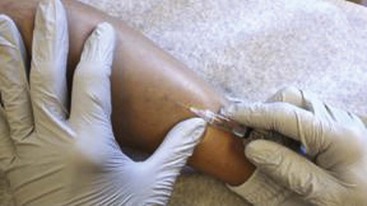
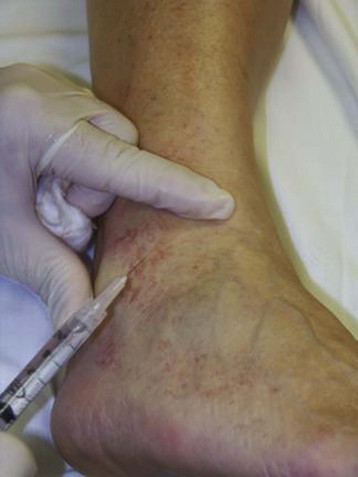
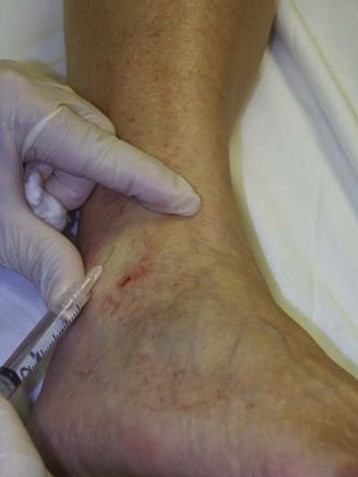
The basic endovascular instrumentation used for the treatment of spider telangiectasias includes needles for access and syringes to deliver the sclerosant to the affected areas. The needles used for telangiectasia are typically 30 gauge, although a 27-gauge butterfly needle may be used sometimes for larger reticular veins. Smaller needles, as small as a 33 gauge, can also be used but they tend to bend too easily when they are penetrating the skin (Fig. 11-9).
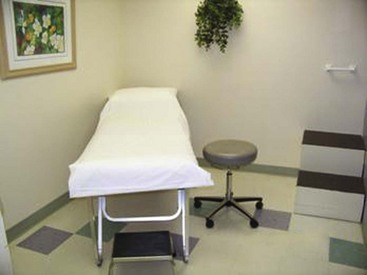
The environment of care for sclerotherapy should include a comfortable table for the patient, a comfortable room temperature, and ample lighting. The treatment table height should permit the physician to sit comfortably on a stool with his or her legs under the table without having to lean over the table and the patient for access. Environmental lighting should be bright and capable of providing adequate indirect illumination without any glare on the patient’s skin (Fig. 11-10).
General supplies would include alcohol swabs, cotton balls, tape, and compression supplies such as Ace wraps or Coban. Patients can supply their own stockings or they can be provided by the practice, which would require keeping a fairly large inventory (Figs. 11-11 and 11-12).
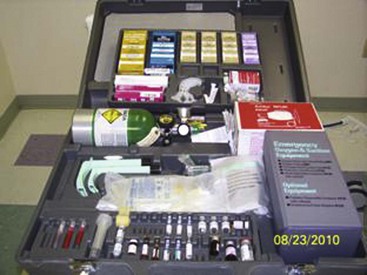
Last, but also most important, is emergency equipment. Fortunately, life-threatening complications are very rare; however, they are always possible. Basic emergency equipment must minimally include oxygen, airway equipment, epinephrine, steroids, and antihistamines (Fig. 11-13).
Imaging
First and foremost are magnifying glasses or loupes. These aids help to visualize the insertion of the needle into the smaller veins, particularly those that are less than 1 mm in diameter. Because loupes typically have 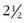 times the magnification, they facilitate good visualization while the needle pierces the skin and enters the vein (Fig. 11-14).
times the magnification, they facilitate good visualization while the needle pierces the skin and enters the vein (Fig. 11-14).
A number of lighting systems are used for visualization of veins to be treated. Vein lights provide visualization of vessels just under the skin that are sometimes too deep for normal visualization. These lights create a “shadow” from the absorption of the blood in the vein. Polarized lights are also used to provide better visualization through the skin (Fig. 11-15).
Syris polarized lights with magnification (Syris, Gray, ME) also provide better visualization through the skin (Fig. 11-16).
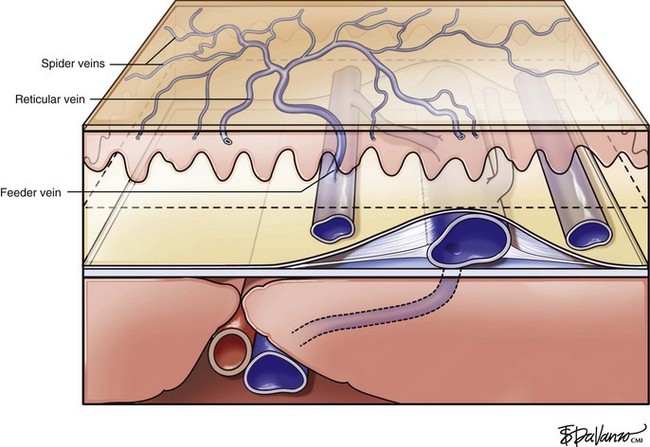
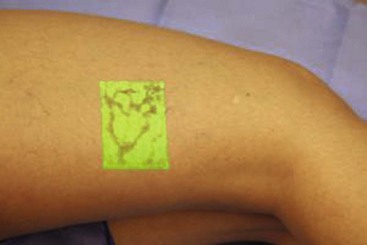
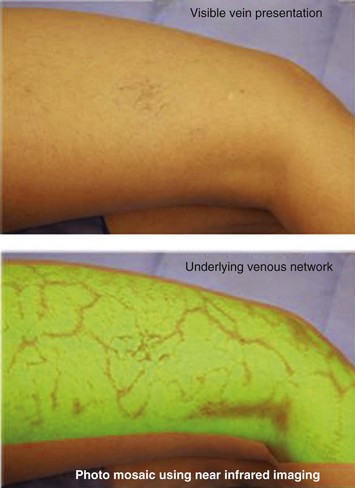
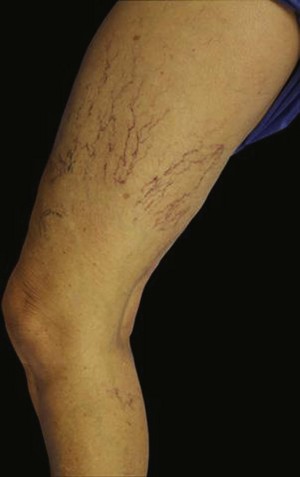
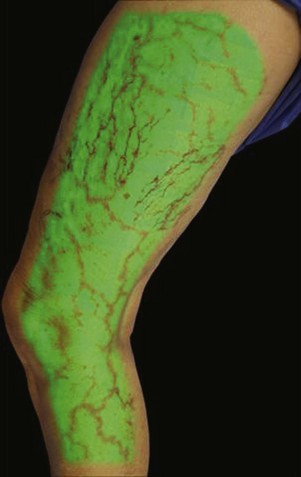
Infrared visualization is also done. Infrared lights allow the practitioner to see veins a few millimeters under the skin because these lights provide infrared images of the hemoglobin contained within the red cells circulating in the vessel that is then projected back onto the skin using the VeinViewer or a camera. This is particularly helpful for identifying “feeding” reticular veins that could be causing the telangiectasias (Fig. 11-17).
Operative Steps
Treatment should begin at the source of reflux, if the source has been determined, or proximal to it if the precise location is not known. In the latter case, the larger veins are treated prior to the treatment of the smaller ones. It can be assumed that the source of reflux is the perigeniculate perforators, located usually just above the knee, for the lateral venous plexus area (Fig. 11-19).
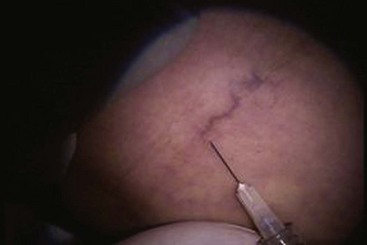
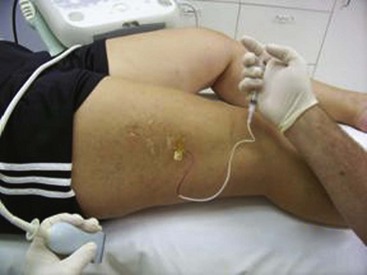
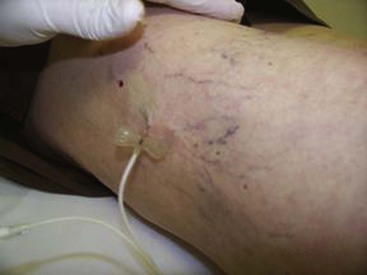
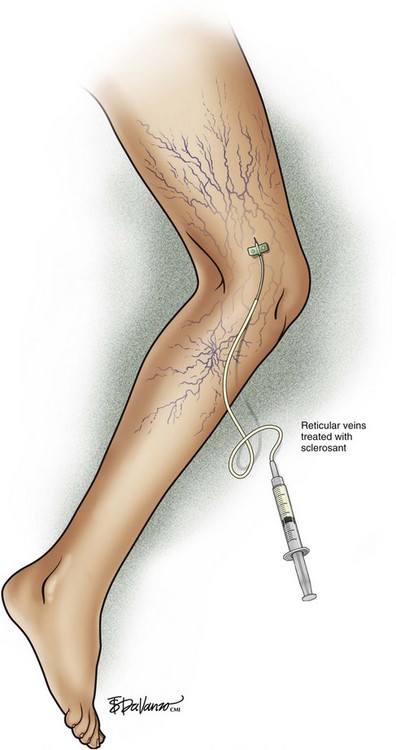
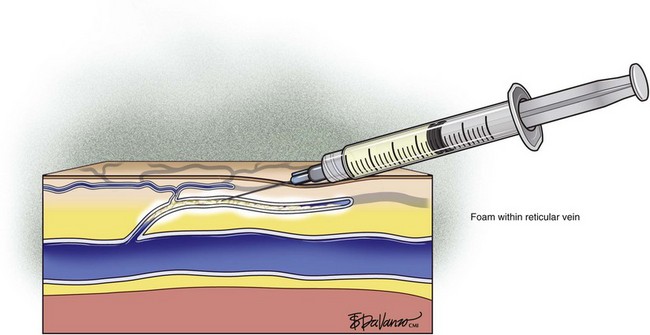
Complete treatment of the reticular feeding veins is performed in a given area before moving to the treatment of the smaller spider telangiectasias in the same area. Sclerosant injected into the feeder vein often travels into the spiders, thus effectively treating both the feeding veins and the spider veins. Access with the needle, as described earlier, is done with aspiration to confirm placement into the larger reticular feeder veins. The method of injection should be smooth and with very little pressure on the plunger. The volume of the injection solution depends on the size of the reticular vein. By definition, reticular veins range from 1 mm to 3 mm in diameter. Using 2 mm as an average, the volume of a 5-cm segment is 0.16 mL. Therefore a 15-cm segment would require 0.5 mL of sclerosant. Imaging with vein lights, a vein viewer, and polarized lights is sometimes very helpful (Figs. 11-20 through 11-22).
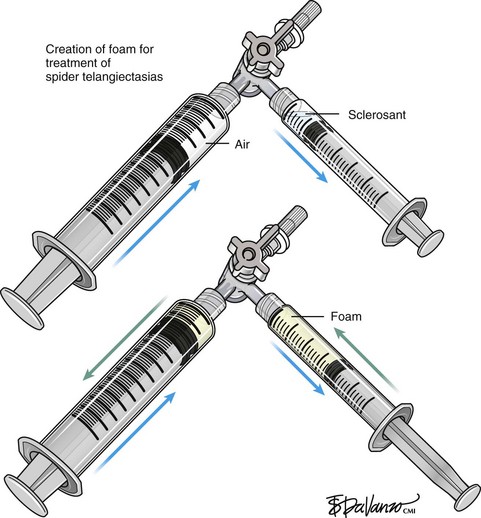
After the injection is stopped, the needle is then held in position for several seconds, up to 30 seconds. This increases the contact time of the sclerosant with the vein wall. The choice and concentration of sclerosant for spider veins are based on the size of the spider vein and practitioner preference, with ranges from 0.05% to 0.25% for STS and from 0.25% to 0.5% for POL, 11.7% HS, and 48% glycerin diluted with lidocaine. The total volume of the injection depends on which type and concentration of sclerosant are being used. For example, the maximum dose for STS is 10 mL of 3%, so if one is injecting a 0.25% solution, the total volume would be 120 mL (Figs. 11-23 and 11-24).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree