Chapter 6 Laser Thermal Ablation
Historical Background
Bruising, transient pain, and induration of the thigh are common adverse events after endovenous laser (EVL) therapy and are most likely caused by laser-induced perforation of the vein wall with extravasation of blood into surrounding tissue.1–3 It is known that conversion of an incompetent vein into a fibrous cord, with subsequent sonographic disappearance, will guarantee permanent occlusion. At the onset of EVL therapy, little was known about the mechanism of action and durability of treatment after intervention with these devices. Studies have indicated that heat-related damage to the inner vein wall leads to thrombotic occlusion of the treated vein.4,5
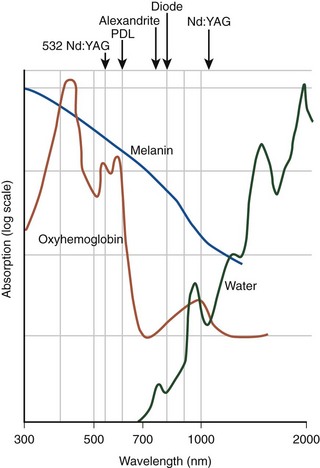
EVL can be classified into hemoglobin-specific laser wavelengths (HSLWs) and water-specific laser wavelengths (WSLWs) (Fig. 6-1). Wavelengths of 808, 810, 940, 980, 1064, 1319, and 1320 nm have been successfully used for great saphenous vein (GSV) ablation4,6–10 and for other superficial axial and perforating veins.11 Hemoglobin and, to a lesser extent, myoglobin in venous smooth muscle cells are the dominant chromophores at the lower end of this range, while at the 1320-nm wavelength range, water dominates as the energy-absorbing molecule.4,7
Published reports suggest that delivery of higher energy is required to effect secure vein closure; however, with increased energy delivery, pain and bruising after treatment are encountered more frequently. After EVL, studies have demonstrated 70% of limbs experience some degree of pain, and 50% require analgesics for pain management.12 Kabnick13 reported an average pain score of 2.6 on a scale of 0 to 5 after EVL. There is increasing focus on reducing perioperative pain and bruising in the field of EVL saphenous ablation. The most recent WSLW to become available is the 1470 nm, which requires less energy delivery for closure, and reports of less pain and bruising postprocedure are beginning to populate the literature.
The WSLWs were developed to target the interstitial water in the vein wall and minimize perforations.7 Two WSLW (1319 and 1320 nm) lasers are in widespread clinical use, and trends in the literature suggest that these longer wavelength lasers may produce fewer side effects than HSLW lasers at comparable linear endovenous energy density (LEED). Two comparisons of different wavelengths with similar delivered laser energy have been performed. One study compared 940-nm and 1320-nm wavelengths in a retrospective analysis, and another compared 810-nm and 980-nm wavelengths in a randomized prospective study.13,14 The two studies demonstrated equivalent safety and efficacy at similar energy dosing. However, EVL performed at comparable LEED with either the 940-nm (HSLW) or the 1320-nm (WSLW) lasers showed a reduction in postoperative pain and bruising with the 1320-nm device. Also, use of less power (5 W) demonstrated a lower rate of side effects than did 8 W with a laser operating at a wavelength of 1320 nm.14 A low rate of pain and bruising was reported after GSV treatment for GSV reflux with the 1470-nm wavelength at 5 W, 30 J/cm.15
Etiology and Natural History of Disease
There remains controversy over the mechanism of action of EVL and most of the investigations were performed with HSLWs. Proebstle found in vitro with an HSLW that the extensive heat damage of the endothelium and the intima came from steam bubble formation and induced full-length thrombotic occlusion of the vein.4 Bush et al.,16 in a histologic study, found that the mechanism of injury with HSLWs is secondary to steam bubbles caused by water evaporation of the blood followed by transmitted heat injury to the tissue. Acutely, there is complete loss of the endothelium and early thrombus formation, followed by the injury response with inflammatory cellular infiltration into the subintimal layers. Eventually, fibroblasts deposit collagen and represent the predominant histologic finding at 4 months postoperatively.
Opposed to the steam bubble theory, Fan and Rox-Anderson17 studied existing histologic reports from studies with excellent closure rates using tumescent anesthesia and found that HSLWs produce a transmural vein wall injury, typically associated with perforations and carbonization. The pattern of injury was eccentrically distributed, with maximum injury occurring along the path of laser contact. The authors concluded that steam production during EVL accounted for only 2% of applied energy dose (i.e., EVL causes permanent vein closure through a high-temperature photothermolytic process at the point of contact between the vein and the laser). Precise mechanism-of-action studies with ample histologic information are lacking with WSLWs.
Although it is not known exactly how much damage to the individual layers of the vein wall is required, it seems that at a minimum, intimal and medial coagulation are necessary for long-term closure. Technically, the depth of penetration of a 940-nm laser beam into blood is limited to approximately 0.3 mm.18 Qualitative analysis with optical coherence tomography in an ex vivo model matched with histologic cross sections showed a symmetric, complete, circular disintegration of intima and media structures, without any transmural tissue defects after radiofrequency ablation (RFA).19 However, with an HSLW laser, pronounced semicircular tissue ablations and complete vessel wall perforations were detected at 35 J/cm LEED. The quantitative analysis demonstrated a significant (p < .0001) increase in intima-media thickness after RF ablation (38% to 67%) and EVL (11% to 46%) and a significant (p < .0001) reduction in vessel lumen diameter (36% to 42%) after RF ablation. No linear correlation could be identified between laser energy level and effects on tissue such as ablation/perforation, media thickening, or vein lumen diameter.22
Weiss20 examined the gross tissue effects and tissue temperatures generated during EVL with an 810-nm diode laser in an in vivo goat model. Using thermal sensors mounted adjacent to the laser optical fiber, they determined the mean temperature at the firing tip was 729° C (peak 1334° C). The intense thermal heating zone appeared to be focally situated around the laser tip; the mean temperature decreased to 231° C and 307° C, 2 mm proximal and distal to the fiber tip, respectively. At 4 mm distal from the fiber tip, the mean temperature decreased further to 93° C. Recently, Disselhoff et al,10 with intravascular temperature measurements in an in vitro system, found that despite the intense heat at the laser tip, the thermal heating zone is predominantly contained within the venous lumen. Zimmet and Min21 demonstrated in a swine model that during EVL with an 810-nm diode laser, ear vein outer wall temperatures ranged from 40° to 49° C. In hind extremity veins, these investigators showed that with tumescent anesthesia, the external vein wall temperatures never exceeded 40° C.
These findings were corroborated in humans by Beale et al.22 when he inserted thermocouples percutaneously, positioned at 3, 5, and 10 mm from a small saphenous vein (SSV), after administration of tumescent anesthesia. He recorded temperatures during EVL with an 810-nm diode laser, using 1-second pulse application at 12 W, and found peak temperatures of 43°, 42°, and 36° C at 3, 5, and 10 mm, respectively, in perivenous tissues.
Pearls and Pitfalls
Dosing
Data on the dose-response relationship between laser energy and durability of vein occlusion began to be published in 2004, when parameters describing the LEED and endovenous fluence equivalent (EFE) were introduced.23 LEED is a linear energy density quantity measured in joules per centimeter, and EFE is an energy parameter that uses a cylindric approximation of the inner vein surface area expressed in joules per centimeter squared.
In a retrospective study,24 recanalization was reported in 20% of cases treated with administration of less than 80 J/cm and was significantly reduced if laser energy exceeded 80 J/cm at a wavelength of 980 nm. However, in a follow-up prospective study by the same author, 9% of veins treated with LEEDs exceeding 80 J/cm unexpectedly recanalized at 6-month follow-up.25 Multiple regression analysis determined that EFE was the most significant predictor of recanalization events and, when exceeding 20 J/cm2, was associated with durable GSV occlusion after 1-year follow-up.26 Another study reported on 129 GSVs and found that 52 J/cm2 EFE was ideal to produce long-term occlusion; this author cautioned that recanalization can occur in patients treated with higher fluence.27
A sliding scale approach has been used at Miami Vein Center since 2002 and has yielded excellent results.11 High rates of vein occlusion and ultimate sonographic disappearance were noted when the thermal dose in each segment of the GSV was tailored to the diameter in that segment. The ranges of energies used fell between 50 J/cm for veins 5 mm in diameter and 120 J/cm for veins 10 mm in diameter at the saphenofemoral junction (SFJ). No increase in complications were seen with any of the higher energy strategies.28
Reports from John Hopkins University on “low-energy” EVL treatment of 34 consecutive GSVs with a 980-nm diode laser at 11 W in continuous mode (mean GSV diameter 12 mm) using mean LEED of 35 J/cm resulted in zero recanalization (100% success) at a mean follow-up of 1 year.29 Interestingly, the same author reported later in the same year that 60 consecutive GSVs demonstrated a reduced treatment success of 95%; the mean follow-up for this series was 6.8 months and mean LEED for successful and failed treatments was 33 J/cm. The mean maximum diameter of successfully treated GSVs was 11 mm, and that for failed treatments was 21 mm (p = .008). The investigators concluded that there were no significant differences in mean unit energy applied for successful, failed, and repeat treatments (p > .05); however, larger GSV diameter was associated with early treatment failures.30
A recent clinical trial indicated that energy density may not be the most important determinant of recanalization. In 471 segments, 11 failures were encountered, including 4 in a group treated with less than 60 J/cm (4%), 2 in a 60 to 80 J/cm group (3%), 4 in an 81 to 100 J/cm group (3%), and 1 in a group treated with more than 100 J/cm (1%). There were no statistically significant differences in failure rates among energy density ranges. The authors concluded that EVL has a low failure rate and that failure rate is not a function of energy density.31
Complications
Significant adverse events reported following EVL include skin burns, sensory nerve injuries, and deep vein thrombosis (DVT). Early experience32 reported skin burn rates as high as 4%; this decreased to almost zero as the use of tumescent anesthesia became the standard of practice. The overall rate for these complications has been shown to be higher in low-volume centers compared to high-volume centers; the rate of skin burns in one series using RFA was 1.7% before and 0.5% after the initiation of the tumescent technique.33 The nerves at highest risk include the saphenous nerve, located adjacent to the GSV below the midcalf perforating vein, and the sural nerve, adjacent to the SSV in the midcalf and lower calf. The most common manifestations of a nerve injury are paresthesias, which are usually transient. The nerve injuries can occur during venous access, the delivery of tumescent anesthesia, or lasering by transfer of thermal energy to perivenous tissues.
Patients treated with EVL, using extraordinarily high rates of energy delivery without tumescent anesthetic infiltrations, demonstrated a high rate of nerve injuries and skin burns.34 However, in a series using RF ablation, delivery of perivenous fluid was thought to be responsible for the low rate of cutaneous and neurologic thermal injuries, where the 1-week paresthesia rate was shown to decrease from 15% to 9% after the introduction of tumescent anesthesia.35 The addition of tumescent anesthesia has been demonstrated to reduce outer vein wall temperatures during EVL and RFA in animal models.36,37
Fortunately, thromboembolic events are uncommon after endovenous thermal ablation, probably because tumescent anesthesia has allowed for speedy procedures, which promotes ambulation immediately after surgery.11 The thrombus extensions at the SFJ seen occasionally after thermal ablation were discussed in Chapter 5.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree