Chapter 61
Iliocaval Venous Obstruction
Surgical Treatment
Yves S. Alimi, Olivier Hartung
This chapter covers three conditions that affect the lower extremities, pelvis, and renal venous drainage: chronic iliocaval venous occlusion; pelvic-renal congestion syndrome, which is due to pelvic varicose veins; and nutcracker syndrome, in which compression of the left renal vein (LRV) causes hematuria and left flank pain, with or without pelvic congestion syndrome.
Chronic Iliocaval Venous Occlusion
Valvular incompetence is the most common cause of chronic venous insufficiency (CVI) of the lower extremities, whereas deep venous obstruction is responsible for the signs and symptoms of venous congestion in less than 10% of patients.1 Although the first successful venous reconstruction in a patient was reported more than 50 years ago by Warren and Thayer,2 the results of open surgical treatment of venous obstruction have been less than satisfactory for many years. Only in the past 2 decades have improvements in diagnosis, patient selection, and surgical technique and the availability of better graft material resulted in more frequent successful implantation of venous bypasses in patients.3 The results of venous grafting, however, are still inferior to those obtained with arterial reconstruction.
Endovascular treatment of iliocaval obstruction has progressed rapidly, and today venous stenting is the primary choice for treatment of benign iliac or iliocaval venous occlusion. Techniques and results of endovenous reconstruction are discussed in detail in Chapter 62. In this section we discuss the indications, patient selection, preoperative evaluation, techniques, and results of open surgical treatment of chronic iliac and iliocaval venous obstruction. We examine causes of graft failure in the venous system and list advantages of different adjuncts used currently to improve the patency of venous grafts. The reader is referred to Chapter 65 for a discussion of vena caval reconstruction in treatment of malignant tumors and to Chapters 158, 159, and 160 for details on venous reconstruction after abdominal or lower limb trauma.
Etiology
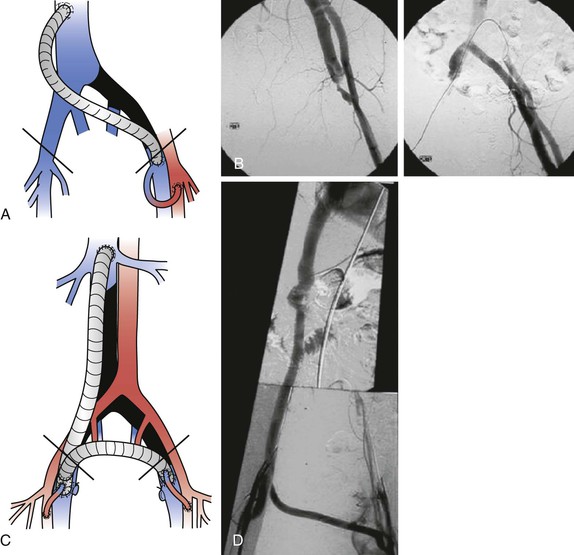
Deep venous thrombosis (DVT) is the most common cause of venous obstruction. In symptomatic patients, recanalization of thrombosed veins in patients with incomplete collateral circulation is inadequate, and in the infrainguinal veins, valvular incompetence develops as a result of destruction of the venous valve leaflets. Venous occlusion may also be due to trauma, irradiation, or external compression of deep veins by retroperitoneal fibrosis4; benign, malignant, primary, or metastatic tumors5–7; cysts; aneurysms; abnormally inserted muscle (popliteal vein entrapment)8,9; and fibrous bands or ligaments (soleal arch syndrome and femoral vein compression by the inguinal ligament10). Compression of the left common iliac vein by the overriding right common iliac artery (May-Thurner syndrome; Fig. 61-1) is a frequently overlooked cause of left iliofemoral venous thrombosis.11–14 May and Thurner observed secondary changes such as an intraluminal web or “spur” in the proximal left common iliac vein in 20% of 430 autopsies. Congenital anomalies, such as membranous occlusion of the suprahepatic inferior vena cava (IVC) with or without associated thrombosis of hepatic veins (Budd-Chiari syndrome),15 aplasia, and hypoplasia of the iliofemoral veins (Fig. 61-2) as in Klippel-Trénaunay syndrome,16,17 can also cause CVI.
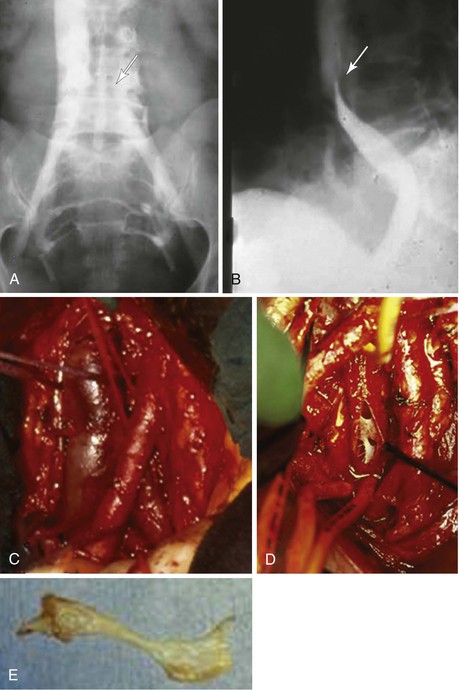
Figure 61-1 Compression of the left common iliac vein by the right common iliac artery (May-Thurner syndrome). A and B, Face (A) and profile (B) phlebograms. The arrow indicates compression of the vein by overriding artery. C, Intraoperative view. D, Intraoperative visualization of the webs inside the left common vein. E, Intraluminal web after removal.
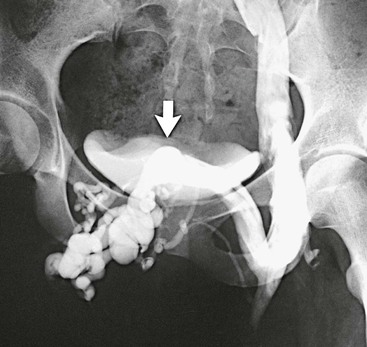
Figure 61-2 Venogram of agenesis of the right iliac vein in a 22-year-old woman. A large suprapubic venous collateral (arrow) is draining the entire right lower extremity. (From Gloviczki P, et al: Klippel-Trénaunay syndrome: the risks and benefits of vascular interventions. Surgery 110:469-479, 1991.)
Preoperative Evaluation
Preoperative evaluation should reveal the cause and functional significance of the deep venous obstruction and the extent and severity of any associated venous incompetence. In at least two thirds of patients with venous outflow obstruction, however, distal reflux secondary to valvular incompetence contributes greatly to the development of CVI.18 For details on the clinical and diagnostic evaluation of patients with CVI, see Chapter 60. The problems inherent in establishing the presence of a pressure-significant venous obstruction are also detailed in Chapter 62. Specific aspects of the evaluation of patients with suspected deep venous occlusion who are candidates for open venous reconstruction are emphasized in this chapter.
History and Physical Examination
The history and physical examination, complemented by examination with a handheld Doppler instrument, should reveal signs and symptoms typical of venous congestion. Patients with venous occlusion have swelling and experience exercise-induced pain in the thigh muscles, referred to as venous claudication. This pain is described as a “bursting” pain in the thigh and sometimes the calf that occurs after exercise and is relieved by rest but more effectively by elevation of the legs.
Signs of CVI, such as edema, varicose veins, skin changes, lipodermatosclerosis, eczema, and ulceration, should be noted. Distended varicose veins are present even in supine patients with CVI, and suprapubic and abdominal wall collaterals develop in patients with pelvic occlusion. Bleeding from high-pressure varicosities is not infrequent. The swollen leg has a cyanotic hue, and bilateral swelling indicates bilateral iliofemoral or vena caval occlusion or systemic disease. In some patients, venous congestion results in hyperhidrosis and significant fluid loss through the skin. Associated chronic high-output or low-output lymphedema (see Chapter 66) may also develop.
The preoperative evaluation should identify risk factors for DVT, including previous DVT or pulmonary embolism, family history of venous thrombosis, obesity, decreased activity level, recent pregnancy, malignant disease, hormonal treatment, hypercoagulability, recent trauma, and previous surgery.
Associated chronic arterial occlusive disease and congenital venous malformations manifested as limb hypertrophy, port-wine stains, and lateral atypical varicose veins (Klippel-Trénaunay syndrome) as well as the presence of a thrill or bruit indicative of an arteriovenous fistula (Parkes-Weber syndrome) should be excluded. Patients with membranous occlusion of the vena cava frequently also have evidence of hepatic failure, Budd-Chiari syndrome, and portal hypertension.15
A simple technique to detect femoropopliteal venous occlusion in the office is the Perthes test. A tourniquet is placed on the proximal aspect of the calf, and the patient is asked to walk. Exercise results in rapid emptying of the superficial vein through patent perforators into a patent deep system. Distention of superficial veins distal to the tourniquet after ambulation indicates deep venous occlusion.
Examination with a handheld Doppler instrument also yields evidence of deep venous occlusion, but the diagnosis should be established by more accurate means, primarily duplex ultrasonography. A complete evaluation to establish the clinical class of venous disease is necessary.
Noninvasive Venous Evaluation
Although duplex scanning and plethysmography are the tests needed to confirm deep venous occlusion and to determine the extent of valvular incompetence and calf muscle pump failure, it is equally important to exclude any abdominal or pelvic disease (tumor, cyst, retroperitoneal fibrosis) with computed tomography (CT) or magnetic resonance imaging (MRI). Outflow plethysmography is useful to confirm functional venous outflow obstruction and to document improvement after treatment.
Ambulatory venous pressure measurements suggest venous hypertension, and measurements of foot-arm pressure differences, as described by Raju, are used to quantitate venous hypertension.19 A resting arm-foot pressure differential greater than 4 mm Hg was considered evidence of significant obstruction justifying venous reconstruction.19 For purposes of testing, exercise consists of 10 dorsiflexions of the ankle or 20 isometric contractions of the calf muscle. In potential candidates for proximal venous reconstruction, femoral and central venous pressure measurements are required in our practice to document the severity of the proximal iliac or iliocaval obstruction. Either a pressure difference of at least 5 mm Hg between femoral and central pressure in a supine patient or a twofold increase in femoral vein pressure after exercise indicates hemodynamically significant proximal stenosis or occlusion.20–24
Contrast-Enhanced Phlebography
In patients being considered for venous reconstruction, detailed contrast-enhanced phlebography is performed. We perform both ascending and descending phlebography to evaluate obstruction and any associated valvular incompetence.22,23 Iliocavography and abdominal venacavography through a brachial approach may also be necessary in some patients to visualize the vena cava proximal to the occlusion. Femoral access is useful not only for performing descending phlebography and iliocavography but also for measuring femoral venous pressure. MR phlebography (see Fig. 61-1) is a promising technique that may further decrease the number of contrast-enhanced radiographic examinations in the future. The technique of contrast-enhanced phlebography is covered in Chapter 20.
Factors Affecting Graft Patency
Grafts placed in the venous system undergo thrombosis more frequently than those implanted for arterial reconstruction. This difference is attributed to several factors. Flow in venous grafts in general is lower than in arterial grafts placed at the same location, primarily because of the collateral venous circulation that developed to compensate for the chronic obstruction. Distal venous obstruction and incompetence further decrease inflow to the graft and thereby potentially contribute to failure.18,23 Pressure in the venous system is low, and grafts can collapse under the increased abdominal pressure or in tightly confined spaces, such as the area under the inguinal ligament and the retrohepatic space, or when they are tunneled through the diaphragm. Many patients with previous DVT lack circulating anticoagulants, such as protein C, protein S, and antithrombin III, and thus have greater thrombogenicity. The thrombogenic surface of the prosthetic graft also increases the risk for graft failure.
Venous Grafts and Adjuncts to Improve Patency
As a result of extensive laboratory efforts made in the past decades,25–38 the patency of grafts implanted in the venous circulation has improved considerably. The availability of large-diameter autologous and prosthetic grafts, use of adjuncts such as a distal arteriovenous fistula, rigid external support of the grafts, perioperative and postoperative anticoagulation, use of perioperative intermittent pneumatic compression pumps, and postoperative surveillance with duplex scanning have all contributed to better patency and improved clinical outcome.
Venous Graft Material
Autologous grafts for the femorofemoral or infrainguinal location have the best chance of long-term success. The great saphenous vein, because of its low thrombogenicity and suitability in terms of size and length, is the best choice when it is available. As a spiral or panel graft, it can also be used for the reconstruction of large veins, although in our experience these grafts do not perform as well for iliocaval reconstruction as they do for superior vena cava replacement (see Chapter 63).22,23 The contralateral superficial femoral vein and the arm veins, especially the basilic-brachial-axillary vein, are other potential sources of autogenous grafts, as are the external and, occasionally, even the internal jugular veins. Harvesting of contralateral deep veins in patients with a history of DVT, however, may be a source of significant added morbidity.
Experiments with cryopreserved femoral vein grafts were promising,39 but one small clinical trial that used cryopreserved veins for valve replacement was not very successful. At 6 months, the actuarial secondary patency rate was 78% ± 13%, and the secondary competency rate of the transplanted valves was 67% ± 16%.40 Data at this point are insufficient to support recommendation of this graft for routine clinical use.
Of the available prosthetic material, expanded polytetrafluoroethylene (ePTFE) has been used most frequently for replacement of large veins.4–7,21–23,41–49 Because of a large diameter, sufficient length, immediate availability, and external ring or spiral support associated with relatively low thrombogenicity, ePTFE grafts continue to be the best choice for prosthetic replacement of large veins.
Experimental work in a canine model showed that seeding the prosthetic grafts with endothelial cells inhibited neointimal hyperplasia and achieved 100% patency of IVC grafts at 100 days.50 This was thought to be related to a higher level of prostaglandin I2 metabolite and a lower level of thromboxane B2 metabolite from the seeded grafts than from spontaneously formed cells. Clinical correlation was also observed in that 10 of 11 grafts were patent during a follow-up period of 6 to 11 years. Similar improvements in arterial beds have been reported with seeding of prosthetic grafts with endothelial cells.51 Endothelial seeding of grafts may improve graft patency and long-term clinical outcomes.
Arteriovenous Fistula
Multiple experiments have confirmed that a distal arteriovenous fistula, first suggested by Kunlin and Kunlin in 1953,30 improves the patency of grafts placed in the venous system.25,28,33,34,52,53 An arteriovenous fistula increases flow and decreases platelet and fibrin deposition in prosthetic grafts (Fig. 61-3).33 Prosthetic grafts have significantly higher thrombotic threshold velocity than autologous grafts and require higher flow to maintain patency.
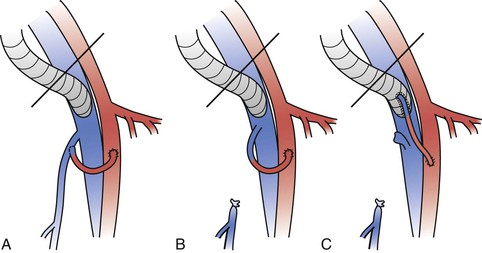
Figure 61-3 Different types of arteriovenous fistulae placed upstream of a ringed polytetrafluoroethylene (PTFE) femoral venous graft. The saphenofemoral vein junction is preserved with the use of either a branch of the great saphenous vein (A) or the main trunk of the great saphenous vein (B). C, The saphenofemoral junction is divided and the main trunk of the great saphenous vein is directly anastomosed to the ringed PTFE graft.
Disadvantages of an arteriovenous fistula include the longer operating time needed to create the fistula and the inconvenience of additional procedures to close the fistula at a later date by means of either endovascular techniques (embolization) or cutdown. A potential side effect is elevated cardiac output caused by high fistula flow; high fistula flow can also defeat the purpose of the operation by increasing venous pressure at the groin and causing venous outflow obstruction. Experimental work in our laboratory has revealed that to avoid deleterious effects on venous outflow from the leg, the optimal ratio between the diameter of the fistula and the graft should not exceed 0.3.52 Elevated intraoperative pressure in the femoral vein after placement of a fistula should be taken as a warning sign, and fistula diameter should be decreased by banding of the conduit.
The configuration and location of the fistula have been the subject of much controversy. A large side branch of the great saphenous vein or the saphenous vein itself can be used to perform one anastomosis only. Most recently, we have placed the venous end of the arteriovenous fistula right onto the hood of the venous graft at the distal anastomosis by using either a 4-mm vein as a free graft (saphenous vein or a large tributary) or a 4-mm ePTFE graft. The advantage of a vein or a polyester (Dacron) fistula is that flow can be calibrated with an electromagnetic flowmeter, and large flows (>300 mL/min) can be avoided.
The arterial anastomosis is usually made to the superficial femoral artery. We place a small polymeric silicone (Silastic) sheet around the fistula to prevent healing and to facilitate dissection of the fistula during a second procedure to occlude it. A 2-0 polypropylene suture is also tied loosely around the fistula with its end positioned in subcutaneous tissue, close to the incision, for later identification; intraoperative duplex scanning facilitates identification of the fistula. Percutaneous closure of the fistula by transcatheter embolization is also an option.
At present, for all prosthetic grafts anastomosed to the femoral vein and all longer (>10 cm) iliocaval grafts, a femoral arteriovenous fistula is added to maintain patency (Fig. 61-4). The fistula is left open for at least 6 months after the operation, but patients without any side effects benefit from long-term fistula flow to prolong patency. For the Palma procedure, we use a fistula selectively and take it down within 3 months after surgery.
Thrombosis Prophylaxis
Intravenous heparin in a dose of 60 units/kg is given before cross-clamping, and anticoagulation is maintained during and after the procedure in most patients. We frequently administer low-dose heparin (500 to 800 units/hr) locally through a small polyethylene catheter in the perioperative period (Fig. 61-5) until complete systemic heparinization is achieved through an increase in the dose, as demonstrated by an increase in the activated partial thromboplastin time to twice normal by 48 hours after surgery. The catheter is then removed, heparinization is continued intravenously through an arm vein or central line or by administration of low-molecular-weight heparin, and the patient begins oral anticoagulation therapy with warfarin.
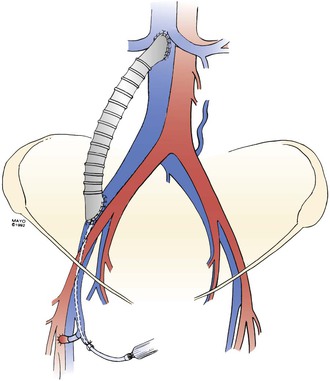
Figure 61-5 A right iliac vein/inferior vena cava externally supported polytetrafluoroethylene graft. Note the arteriovenous fistula on the right side of the groin and a 20-gauge catheter introduced through a tributary of the saphenous vein for perioperative infusion of heparin. (From Gloviczki P, et al: Reconstruction of large veins for nonmalignant venous occlusive disease. J Vasc Surg 16:750, 1992. With permission from the Mayo Foundation.)
An intermittent pneumatic compression pump, leg elevation, elastic bandages, and early ambulation are also used in the perioperative period to improve the success of venous reconstruction.54 The patient is fitted with 30 to 40 mm Hg graduated elastic compression stockings before discharge. Warfarin is continued in patients with autogenous grafts for at least 3 months; in most patients with prosthetic grafts or with an underlying coagulation abnormality, however, oral anticoagulation is maintained indefinitely.
Graft Surveillance
Intraoperative duplex scanning is performed in most patients to ensure patency, good flow, and lack of thrombus deposition. Direct pressure measurements with and without graft flow are made before wound closure in every patient to document the hemodynamic benefit. In venous or polyester conduits, fistula flow can be measured and calibrated with an electromagnetic flowmeter. If flow is higher than 300 mL/min, banding of the fistula should be considered.
On the first postoperative day, we perform contrast-enhanced phlebography through a catheter positioned at the distal anastomosis of the graft (see Fig. 61-5). Any stenosis or thrombosis detected at this time is revised during a reoperation. Postoperatively, the grafts are assessed by duplex scanning at 3 and 6 months and then twice yearly afterward. Outflow plethysmography is also performed to document improvement. In symptomatic patients, however, contrast-enhanced phlebography is usually performed to exclude graft stenosis.
Indications for Surgical Treatment
Failure of conservative management or endovascular treatment of symptomatic venous obstruction is a potential indication for open surgical reconstruction. The severity of the venous stenosis, the location and length of the venous occlusion, the age of the thrombus, the nature of any external compression (e.g., tumor, retroperitoneal fibrosis), the presence of any underlying malignant disease, and the risks associated with a surgical intervention all play a role in determining whether to attempt endovascular or direct surgical intervention.
Because of the reported success of endovascular treatment,55–58 our policy has changed considerably in recent years. Today we attempt endovascular treatment, alone or in combination with thrombolysis, surgical thrombectomy, or excision of old thrombus from the femoral veins, first before surgical bypass is recommended.
Proper patient selection is important, and patients with severe infrainguinal postthrombotic disease and valvular incompetence will have a decreased chance of success. It has been shown in a Mayo Clinic series of seven patients who underwent bypass grafting in the presence of moderate or severe venous incompetence that four had graft occlusion, whereas in four patients without reflux, three had patent grafts with clinical improvement.23 Postoperative surveillance, anticoagulation, and creation of an arteriovenous fistula all predict better long-term results.
Surgical Procedures
Saphenopopliteal Bypass
Although this chapter is devoted to iliofemoral venous reconstruction, it is worthwhile to briefly mention saphenopopliteal bypass, which is designed for chronic occlusion of the femoral or the proximal popliteal vein. This operation was first advocated by Warren and Thayer2 in 1954 and reintroduced by Husni59 and May60 in the 1970s (May-Husni operation). A single distal anastomosis is performed, usually end to side, between the mobilized and divided ipsilateral saphenous vein and the popliteal vein. A temporary arteriovenous fistula can be constructed at the ankle between the posterior tibial artery and one of the paired posterior tibial veins or even with the saphenous vein at the ankle in an end-to-side fashion.44,61 An alternative to the traditional technique of saphenous vein transposition is popliteofemoral or popliteosaphenous bypass (Figs. 61-6 and 61-7) with a free graft that uses either the ipsilateral or the contralateral saphenous vein or an arm vein.
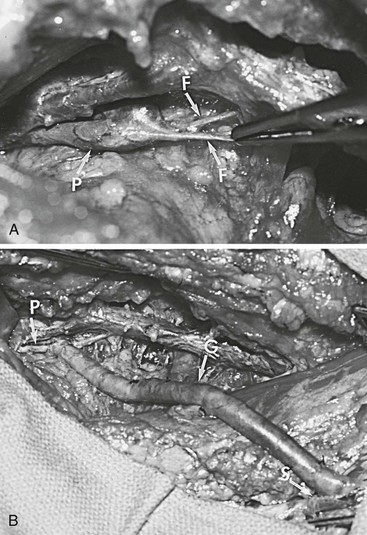
Figure 61-6 A, Klippel-Trénaunay syndrome with atresia of the right femoral vein in a 20-year-old patient. F, fibrous bands replacing the superficial femoral vein; P, popliteal vein. B, Intraoperative photograph of the contralateral saphenous vein graft interposed between the patient’s popliteal and proximal great saphenous veins. G, Vein graft; P, popliteal vein; S, great saphenous vein. (From Gloviczki P, et al: Klippel-Trénaunay syndrome: the risks and benefits of vascular interventions. Surgery 110:469-479, 1991.)
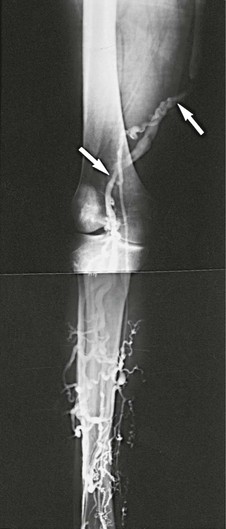
Figure 61-7 Venogram performed 5 months after a popliteosaphenous vein interposition graft. Arrows indicate proximal and distal anastomoses of the patent graft. Note the absence of the right femoral vein. The popliteosaphenous vein has been patent and competent for 8 years, 6 months. (From Gloviczki P, et al: Klippel-Trénaunay syndrome: the risks and benefits of vascular interventions. Surgery 110:469-479, 1991.)
The original May-Husni transposition is rarely performed now, and popliteofemoral bypasses are also uncommon. In 9 reported series involving 218 patients, functional improvement was reported in 77% of cases.44,61–68 In an earlier review of 59 operations, Smith and Trimble reported clinical success in 76% of patients.69 With the exception of one series,67 cumulative patency rates were not reported. Crude patency rates at variable follow-up intervals ranged from 5% to 100%, but only four of the nine studies reported on late imaging of the grafts to ascertain patency.44,61–68
Coleman and associates70 have recently published a series of 17 patients (median age, 41 years; range, 23-69 years) who underwent a saphenopopliteal bypass for chronic edema and venous claudication in all patients. Three patients had a concomitant arteriovenous fistula, created at the time of bypass to enhance inflow, and three patients underwent concomitant femoral-femoral venous bypass. After a median follow-up of 103 months (range, 3-271 months), 82% of patients experienced near or complete resolution of venous claudication. Three of the five patients with venous ulceration healed their wounds (67%). Of the 16 patients who underwent Duplex scan follow-up, primary patency after a median follow-up of 103 months was 56%, primary-assisted patency was 69%, and secondary patency was 75%.
Cross-Pubic Venous Bypass (Palma Procedure)
Initially described about 50 years ago by Palma and Esperon71,72 in Uruguay and popularized by Dale65 in the United States, the Palma procedure has remained a useful technique for venous reconstruction in patients with proximal outflow obstruction (Fig. 61-8). This operation, which was designed for patients with chronic unilateral iliac vein obstruction of any cause, requires a normal contralateral iliofemoral venous system to ensure venous drainage. Results have been better in patients with intact inflow, when the affected limb has no infrainguinal obstruction or deep venous incompetence.
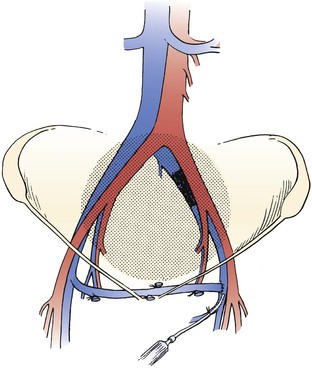
Figure 61-8 Left-to-right femorofemoral venous bypass (Palma procedure) for left common iliac vein obstruction. A small polyethylene catheter can be placed through a side branch of the greater saphenous vein for immediate perioperative heparinization to improve chances of patency. (From Rhee RY, et al: Iliocaval complications of retroperitoneal fibrosis. Am J Surg 168:179, 1994.)
We favor this operation, especially in young women with residual chronic iliac vein occlusion after acute left iliofemoral venous thrombosis that developed as a result of May-Thurner syndrome. The operation is indicated in patients who are not candidates for iliac vein stenting or for whom previous endovascular procedures have failed.
Technique.
For the original operation, the contralateral great saphenous vein is used (Fig. 61-9). Preoperative imaging of the potential graft conduit with phlebography or duplex scanning is recommended because varicose saphenous veins or veins smaller than 4 mm in diameter have a poor chance of long-term success.
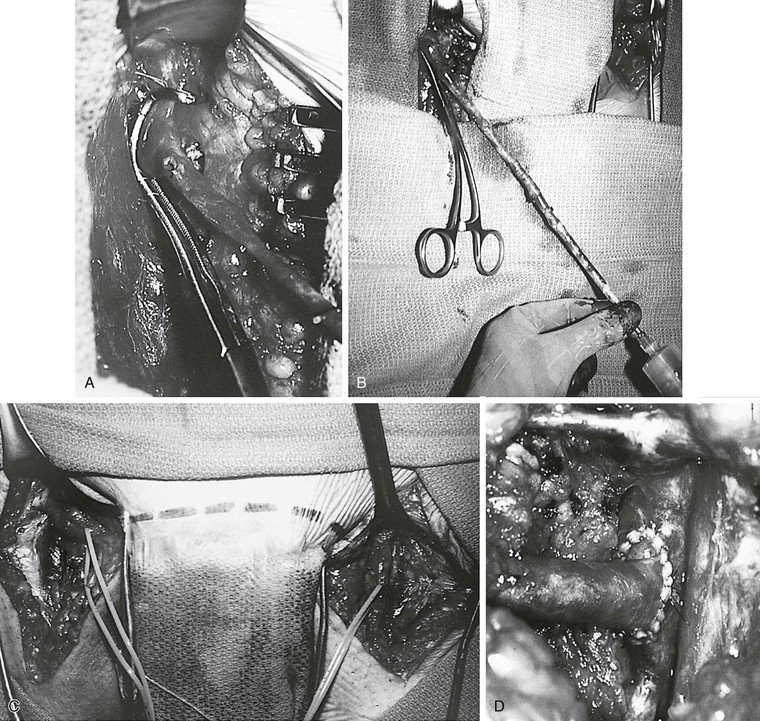
Figure 61-9 A, Technique of the Palma procedure. The contralateral saphenous vein is dissected, and a vascular clamp is placed on the common femoral vein. B, The vein is distended with papaverine solution. C and D, The saphenous vein is tunneled to the left groin and anastomosed end to side to the common femoral vein. A femoral arteriovenous fistula was constructed and encircled with a Silastic sheath for easy identification for takedown 3 months later.
Endoscopic harvesting of a 25- to 30-cm segment of the contralateral saphenous vein ensures an excellent cosmetic result; otherwise, the vein can be dissected through two or three small skin incisions.
After ligation and division of all tributaries, the graft is gently distended with a heparinized papaverine solution and tunneled to the contralateral groin in a suprapubic, subcutaneous position. Dissection of the femoral vein on the affected side should be minimal; usually, just the anterior and lateral vein wall is freed for proximal and distal clamps or for a side-biting clamp to occlude the femoral artery for the anastomosis. Excision of intraluminal fibrous bands after venotomy may be needed. The anastomosis between the saphenous and femoral veins is performed in an end-to-side fashion with standard atraumatic vascular surgical technique. If the vein is small, interrupted 5-0 or 6-0 sutures are preferred to permit later dilatation of the vein and to avoid “purse-stringing” of the venous anastomosis. A small catheter can be placed through a tributary of the ipsilateral saphenous vein for immediate low-dose heparinization and postoperative phlebography (Fig. 61-10). A temporary arteriovenous fistula can also be placed to improve flow and aid in achieving early patency.
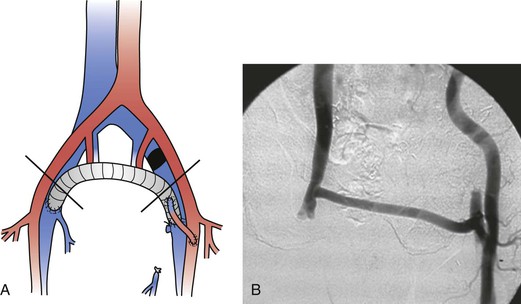
Figure 61-10 A, A femorofemoral ringed polytetrafluoroethylene graft with a left femoral arteriovenous fistula for left iliac vein occlusion. B, Postoperative fistulography.
If the traditional transposition results in significant kinking of the saphenous vein at the contralateral groin, free vein grafting with excision of the saphenous vein along with a small rim of the common femoral vein and reimplantation after a 180-degree turn should be considered. For autologous graft material, the contralateral or even the ipsilateral saphenous vein (with lysis of any competent valves) or an arm vein can be used.
Kistner reported another ingenious idea for an autologous suprapubic graft.46 Through a retroperitoneal approach, the ipsilateral iliac vein is mobilized to the site of the occlusion. The internal iliac vein is ligated and divided to provide adequate length for the conduit. The common iliac vein is then divided and used for suprapubic subcutaneous transposition, with a single anastomosis performed between the iliac and the contralateral femoral veins. This operation can be performed only in patients with short proximal common iliac vein obstruction.
When suitable autologous conduit is not available, an 8- or 10-mm, externally supported ePTFE graft is the best alternative.44,74
Results.
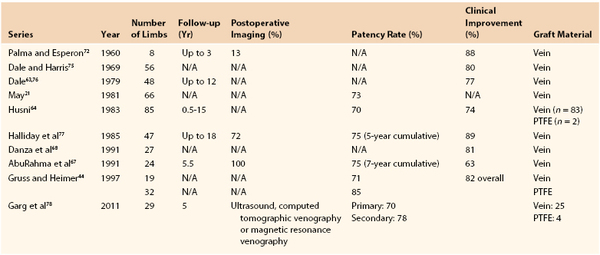
Analysis of the results of 433 operations published in 10 series revealed clinical improvement in 63% to 89% of patients* (Table 61-1). Reported patency rates ranged between 70% and 85%, but follow-up periods were variable and objective graft assessment by imaging was rarely performed in all patients. The largest series, 85 crossover venous bypasses, was reported by Husni.64 At the last follow-up visits ranging from 6 months to 15 years after surgery, 47 of 67 grafts were patent. Results in this study were better when a temporary distal arteriovenous fistula was used. The patency rate of grafts implanted for extrinsic compression of the iliac vein without distal disease was 100%, as opposed to 67% in patients with postthrombotic syndrome.
In a review of 50 consecutive operations performed in 47 patients, Halliday and associates reported a cumulative patency rate of 75% at 5 years confirmed by phlebography.77 Clinical improvement in this series was seen in 89% of patients. The cumulative patency rate in another series of 24 patients was 75% at 7 years, but the standard error in these calculations was high because of the low number of patients studied. Recently, the 5-year primary and secondary patency rates of this Mayo Clinic series including 29 femorofemoral bypasses (Palma vein, 25; polytetrafluoroethylene, 4) were 70% (CI 48 %-85%) and 78% (CI 55%-90%). The endoscopic vein harvest (n = 10) group had lower primary patency (44% vs 86% at 1 year; relative risk, 4.9; P = .04) compared with the open group (n = 15), although secondary patency in the endoscopic vein harvest group was 65% at 1 year and was not significantly different from the open harvest group (relative risk, 1.96; P = .44).78
Danza and colleagues reported somewhat better results with use of the saphenous vein as a free graft than with saphenous transposition.68 Of 19 patients who underwent free saphenous vein grafting, 84% experienced either symptomatic relief or improvement versus 75% of 8 patients who underwent transposition. Gruss24 and Gruss and Heimer44 reported good results with ePTFE grafts. Although the length of follow-up was not mentioned, long-term patency was noted in 22 of 26 grafts. On the basis of these results and those of others, Gruss recommends using externally supported ePTFE grafts with an arteriovenous fistula for all cross-femoral venous bypasses.24
Plethysmographic evidence of outflow obstruction was an independent predictor of clinical outcome in the report by AbuRahma and coworkers.67 Of their patients, 88% undergoing the Palma procedure who had abnormal preoperative maximum venous outflow showed significant clinical improvement, whereas 86% of those with normal preoperative maximum venous outflow had no improvement after surgery.
Prosthetic Femorocaval, Iliocaval, or Inferior Vena Caval Bypass
Anatomic in-line iliac or iliocaval reconstruction can be performed for (1) unilateral disease when autologous conduit for a suprapubic graft is not available or (2) bilateral iliac, iliocaval, or IVC occlusion. Extensive venous thrombosis (not infrequently occurring after previous placement of a vena caval clip), tumors, and retroperitoneal fibrosis not responding to nonoperative therapy are potential indications. Failure of previous endovascular attempts and occlusion after placement of multiple stents have also been indications for bypass.
Technique.
The femoral vessels (for the arteriovenous fistula or for the site of the distal anastomosis) are exposed at the groin through a vertical incision. The iliac vein or the distal segment of the IVC is exposed with a right oblique flank incision through a retroperitoneal approach. The vena cava at the level of the renal veins is best exposed through a midline or a right subcostal incision. The ascending colon is mobilized medially, and the vena cava is exposed retroperitoneally. The infrarenal IVC is reconstructed with a 16- to 20-mm graft, the iliocaval segment usually with a 14-mm graft, and the femorocaval segment with a 10- or 12-mm ePTFE graft. The arteriovenous fistula is constructed first in patients who undergo a long iliocaval bypass (see Fig. 61-5).
A short iliocaval bypass with a significant pressure gradient can be performed without an arteriovenous fistula, as discussed earlier. Reconstruction of the vena cava with a straight PTFE graft, if inflow is good, is also usually performed without an additional arteriovenous fistula. In patients who undergo femorocaval bypass, we perform the proximal and distal anastomoses of the bypass first and then create the arteriovenous fistula before opening up the circulation through the graft. As discussed previously, we generally use a tributary of the greater saphenous vein. The operation is performed with the patient fully anticoagulated with intravenous heparin, and at the end of the operation, as mentioned, a small polyethylene catheter is placed at the level of the distal anastomosis to infuse low-dose heparin (500 units/hr).
Results.
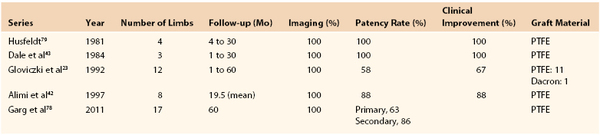
Experience with femorocaval or iliocaval bypass is limited, and only a few series are available (Table 61-2).23,42,43,53,79 In a Mayo Clinic series of 17 such bypasses, primary and secondary patency rates at 5 years were 63% and 86%, respectively (Figs. 61-11 to 61-15).53
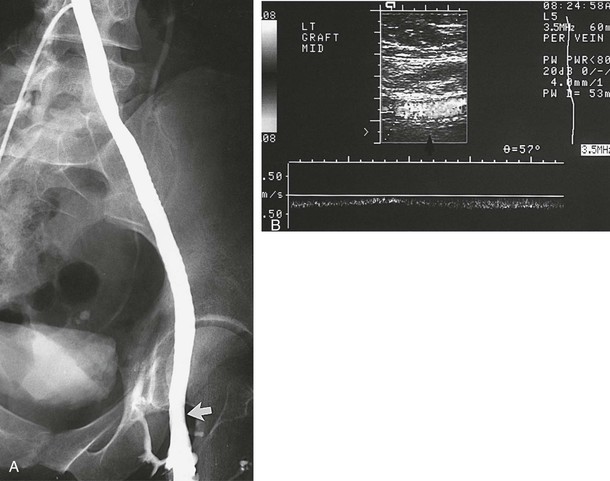
Figure 61-11 A, A venogram 1.6 years after implantation in a 36-year-old woman confirms a widely patent 10-mm polytetrafluoroethylene graft. The arrow indicates the site of the end-to-end femoral anastomosis. B, This patient is free of symptoms 10 years after the operation as shown by duplex ultrasound evidence (arrow) of graft patency.
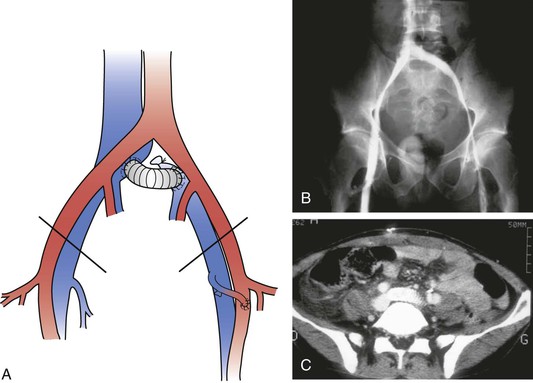
Figure 61-12 A, A short left iliocaval ringed polytetrafluoroethylene graft with a left femoral arteriovenous fistula. B, Bilateral phlebography showing a patent graft 3 months after surgery. C, CT angiography showing a patent graft 6 months after surgery.
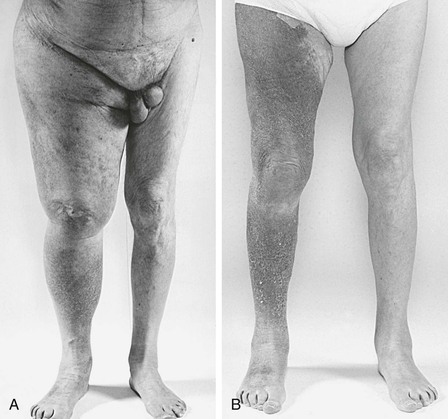
Figure 61-13 A, Preoperative photograph of an 81-year-old patient with severe right lower extremity swelling and massive transudation of fluid as a result of right common iliac vein obstruction (see the venogram in Fig. 60-12). B, A photograph 9 months after the operation confirmed excellent clinical results.
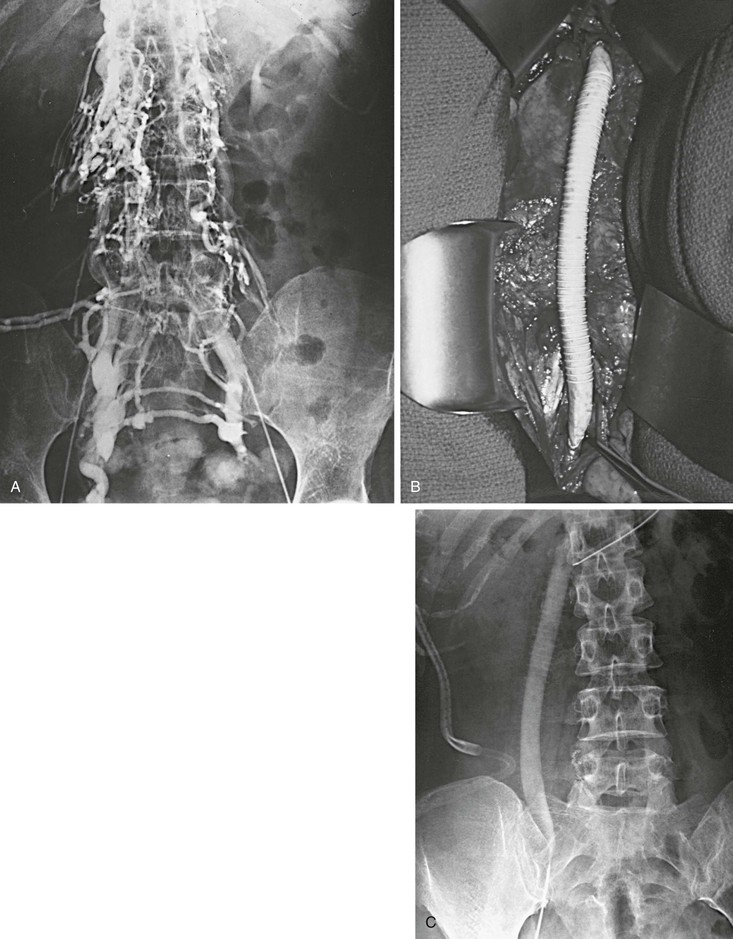
Figure 61-14 A and B, Extensive iliocaval obstruction in a 47-year-old patient who underwent right iliocaval bypass with a right saphenous vein/common femoral artery arteriovenous fistula. C, Postoperative venogram showing a patent graft.
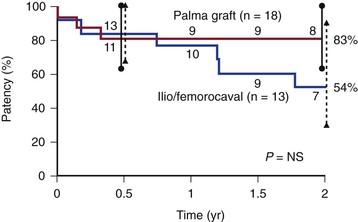
Figure 61-15 Cumulative secondary patency rates of saphenous vein crossover bypass grafts (Palma procedure) and expanded polytetrafluoroethylene iliocaval and femorocaval bypass grafts. (Redrawn from Jost CJ, et al: Surgical reconstruction of iliofemoral veins and the inferior vena cava for nonmalignant occlusive disease. J Vasc Surg 33:320-328, 2001.)
Alimi and colleagues reported the results of eight iliac vein reconstructions with femorocaval or iliocaval bypass for both acute and chronic obstruction.42 In the four patients with chronic obstruction, three grafts were patent at last follow-up. Eklof and associates observed only one occlusion in five grafts monitored for 14 to 22 months after surgery in which bypass was combined with venous thrombectomy for acute DVT.80 Dale and coworkers implanted the first iliocaval bifurcated PTFE graft in 1984,43 and Gloviczki et al reported placement of a bifemorocaval bypass with bilateral arteriovenous fistulae in 1988.22
The largest experience with PTFE reconstruction of large veins was presented by Sottiurai and colleagues (unpublished data).81 Of 56 grafts, 52 (93%), including 13 femorocaval and 26 femorofemoral grafts used for a variety of central vein reconstructions, were reported to be patent at 1 year after implantation. An arteriovenous fistula in this study was constructed with a saphenous vein between the adjacent artery and the graft. The site of the fistula was 2 cm proximal to the distal heel of the graft, where an ovoid 4 × 10-mm defect was cut in the graft wall to standardize flow through the fistula. Dale and colleagues did not favor the use of an arteriovenous fistula with PTFE grafts43; like Sottiurai and associates,81 however, we believe that all femorocaval or longer iliocaval grafts require a distal arteriovenous fistula to maintain patency. Our policy now is to keep the fistula patent as long as possible.52,53
Combined Endovascular and Open Reconstruction
The combination of long iliac vein occlusion with chronic thrombosis of the common femoral vein precludes effective venous stenting. In these patients, open surgical thrombectomy of the common femoral vein with excision of old thrombus and recanalized intimal strands can be combined with intraoperative iliac or iliofemoral vein stenting. We have performed these procedures in the endovascular operating room, which permits the combination of excellent imaging for venous stenting and an optimal operating room environment for the open procedure. A saphenous vein or bovine pericardial patch can be used to close the defect after thrombectomy in the femoral vein (Fig. 61-16). Long-term results of these procedures are not yet available; however, the 2-year patency of these hybrid reconstructions was recently published in a Mayo Clinic series,82 including four patients with stent placement extended into the common femoral vein patch and five patients with the stent terminated proximal to the common femoral vein patch. All of the latter had an early failure, and two of them had common femoral vein stent extension at time of revision. Two-year secondary patency in patients with common femoral vein extension (n = 6) was 67% compared with 0% without common femoral vein stent (n = 3).
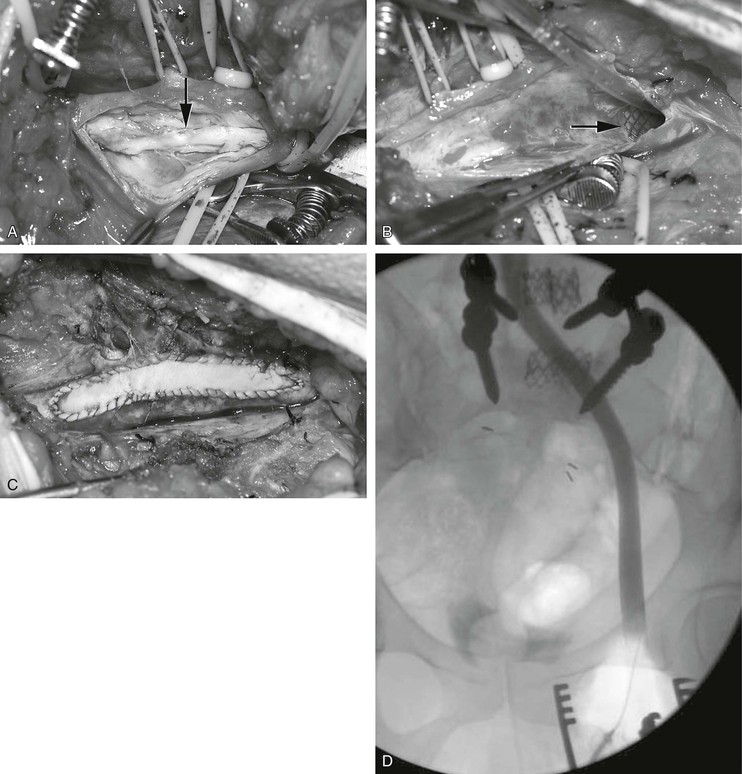
Figure 61-16 Combined endovascular and open reconstruction for chronic iliofemoral venous occlusion. A, Note the old recanalized thrombus (arrow) in the common femoral vein. B, The old thrombus was excised and the iliofemoral vein was stented with Wallstents (arrow). C, The femoral vein was closed with a bovine pericardial patch. D, Postoperative venogram confirming a widely patent iliofemoral vein.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree