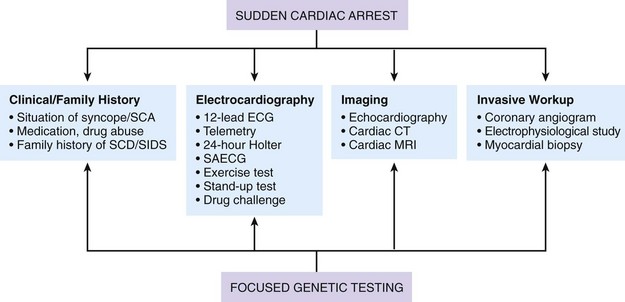
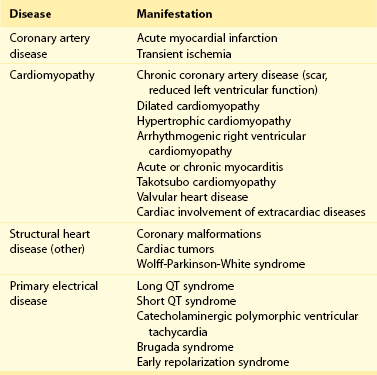
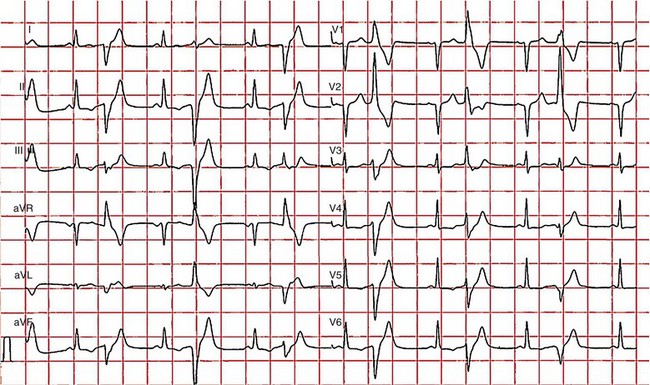
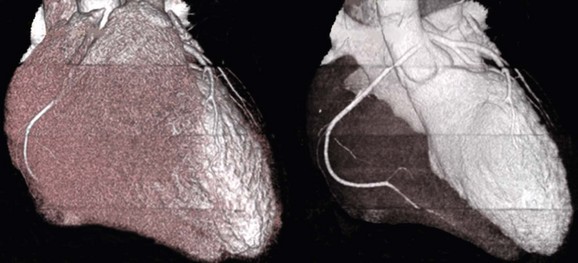
97 Ventricular fibrillation in patients without structural heart disease is rare. Ninety to ninety-five percent of individuals with ventricular fibrillation reveal underlying structural heart disease. No structural heart disease can be identified in only 5% to 10% of patients. According to the results of the Cardiac Arrest Survivors With Preserved Ejection Fraction Registry (CASPER) among patients with normal left ventricular function, a causal diagnosis for ventricular fibrillation can be found in 56%.1 Most diagnoses were primary electrical diseases (catecholaminergic polymorphic ventricular tachycardia [CPVT], long QT syndrome, early repolarization syndrome, and Brugada syndrome [69%]). In 31% of patients, a subtle structural heart disease (i.e., coronary spasm, subclinical arrhythmogenic right ventricular cardiomyopathy and myocarditis) was identified. In addition, idiopathic ventricular fibrillation (IVF) was diagnosed in 44% of patients with ventricular fibrillation without structural heart disease.1 Patients with IVF had a mean age of 40 to 45 years.1,2 The diagnosis of IVF is based on the exclusion of currently known structural and primary electrical heart diseases following a complete noninvasive, invasive, and genetic workup. Over time, the group of patients diagnosed with IVF has declined because more entities have been identified that were previously classified as IVF. Primary electrical diseases with characteristic electrocardiographic patterns such as long QT syndrome, Brugada syndrome, CPVT, short QT syndrome, and early repolarization syndrome are to be ruled out before the diagnosis of idiopathic VF can be established.3 In addition to distinct electrocardiographic patterns, new genetic mutations encoding for cardiac ion channels or structural proteins could be associated with IVF.4,5 The ECG in IVF is usually normal or has at the most subtle unspecific changes. Viskin et al.6 showed that male patients with IVF had slightly shorter QTc intervals compared with healthy controls. Whether this finding is clinically relevant needs to be shown in the future.6 An early repolarization pattern in the inferolateral leads was associated with an increased risk for sudden cardiac death7; this finding was confirmed in large population-based studies.8 Early repolarization seems to indicate a higher risk in patients without structural heart disease and in patients with acute or chronic coronary artery disease.9,10 Because of the high prevalence of early repolarization in the general population, it remains unclear how to identify those who are at increased risk of sudden death.11 J waves syndrome is discussed in detail in Chapter 96). Specific premature ventricular contractions (PVCs) can trigger polymorphic ventricular tachycardia or ventricular fibrillation. These PVCs are typically shortly coupled and arise from the right ventricular outflow tract (RVOT) or the Purkinje system.12,13 In patients without organic heart disease, the most common site of origin of PVCs and ventricular tachyarrhythmias is the RVOT. The morphology of ventricular tachycardias usually resembles a left bundle branch block pattern with an inferior axis. The clinical course of these patients is considered predominantly benign; however, benign PVCs originating from the RVOT can initiate ventricular fibrillation.14 Risk factors for potentially malignant RVOT electrical activity appear to be a history of syncope, rapid ventricular tachycardias (rates of more than 230 beats/min), frequent PVCs (more than 20,000 per day), and ventricular ectopy with a short coupling interval.15 However, a comparison of patients with benign PVCs and patients with PVCs that can induce IVF also shows a significant overlap of clinical parameters, such as lower number of PVCs and longer coupling intervals (more than 320 ms) and QRS duration of PVCs.14 Thus, identification of patients with potentially malignant ventricular extrasystoles still remains a challenge. The Purkinje system is another source of triggers initiating IVF (Figure 97-1). Haissaguerre et al.16 showed that PVCs with narrow QRS morphology and right or left bundle block pattern originating in the area of the left or right Purkinje system are involved in the induction of ventricular fibrillation.16 This study confirmed the role of the Purkinje system in the initiation of IVF, although the exact mechanisms remain unknown.13 Figure 97-1 The 12-lead electrocardiogram of a female patient with idiopathic ventricular fibrillation. Multiple premature ventricular contractions (PVCs) from the left Purkinje system could be recorded. After implantation of an implantable cardioverter defibrillator (ICD), PVCs were ablated following multiple ICD discharges. The diagnosis of IVF can be established only if structural and electrical cardiac abnormalities have been excluded. Thus, a complete systematic cardiologic workup is mandatory (Figure 97-2). Besides noninvasive and invasive examinations, clinical and family history provides considerable information on potential underlying diagnosis. In the setting of absent structural heart disease, syncope or sudden cardiac arrest during exercise or stress indicate long QT syndrome or CPVT. Sleep-related sudden death is associated with long QT3 syndrome or Brugada syndrome. Furthermore, information on medication provoking QT prolongation (www.qtdrugs.org) or the Brugada phenotype (www.brugadadrugs.org) and fever-related sudden death may also be helpful.17 Coronary artery disease accounts for the largest number of sudden deaths of cardiac etiology; therefore, ischemia must be excluded in the workup for IVF. Coronary angiography and cardiac computed tomographic scan are the methods of choice to exclude acute myocardial infarction or coronary anomalies (Figure 97-3). Because ventricular fibrillation may be the first manifestation of coronary artery disease, coronary angiography should be considered even in patients younger than 35 years. Figure 97-3 Cardiac computed tomographic three-dimensional reconstruction of a 23-year-old man who was resuscitated for ventricular fibrillation. During a coronary angiogram, an anomalous origin of the right coronary artery form the left aortic sinus was noted. Cardiac computed tomography confirmed this finding. The right coronary artery runs between aorta and pulmonary trunk (right without right ventricle). Blood tests are needed for exclusion of electrolyte imbalance or drug intoxications triggering ventricular tachyarrhythmias.18 Echocardiography should be repeated during follow-up because cardiopulmonary resuscitation often leads to temporary impairment of left ventricular function and ECG changes, which can be misdiagnosed. Furthermore, in dilative cardiomyopathy, HCM, or ARVC, the first clinical phenotype might be ventricular fibrillation before impairment of LV function or structural abnormalities can be detected.19 Ventricular tachyarrhythmias or ventricular fibrillation have been reported in left apical ballooning (Takotsubo cardiomyopathy) as a newly recognized form of cardiomyopathy.20 Cardiac magnetic resonance imaging can be applied to rule out ARVC.21 Evidence of late gadolinium enhancement indicates a scar caused by coronary artery disease, myocarditis, ARVC, or hypertrophic cardiomyopathy. Extracardiac diseases with potential cardiac involvement, such as sarcoidosis, Fabry disease, or neurodegenerative disorders, should also be excluded. Recently, a new entity has been described: sudden unexpected death in epilepsy (SUDEP). Patients with epilepsy can die suddenly in the absence of structural heart disease with or without the evidence of seizure. The mechanism of SUDEP is not fully understood. Cardiac arrhythmias, postictal cardiomyopathy, seizure-related respiratory failure, and alternated autonomic function are discussed in literature. Ventricular fibrillation might be one specific cause in SUDEP. Specific mutations in genes encoding also for channelopathies like Brugada syndrome and long QT syndrome have been identified in SUDEP.22,23 Thus, a link between ion channelopathies in the heart and the brain exists and could indicate ventricular tachyarrhythmias as one cause for SUDEP (Table 97-1).
Idiopathic Ventricular Fibrillation
Historical Perspective
Electrocardiographic Phenotype and Triggers of Idiopathic Ventricular Fibrillation
Diagnostic Evaluation
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Thoracic Key
Fastest Thoracic Insight Engine