It has become clear that hypertrophic cardiomyopathy is a genetic disease. In fact, it is the most common inherited cardiovascular disorder, afflicting one of every 500 individuals. From a molecular perspective, hypertrophic cardiomyopathy is caused by mutations in at least one of the ten proteins of the cardiac sarcomere. Despite our ever increasing understanding of the molecular etiologies of this complex disorder, the clinical diagnosis of hypertrophic cardiomyopathy remains within the realm of the echocardiographer. The most common clinical manifestation of hypertrophic cardiomyopathy is left ventricular outflow obstruction. However, diastolic heart failure and mitral regurgitation are also prominent features of this disease that can be evaluated with echocardiographic techniques in patients of all ages.
This chapter reviews the common echocardiographic features displayed by patients with hypertrophic cardiomyopathy. We will also outline a classification and examination strategy that will not only detect the presence of the disease, but also assist with planning both medical and surgical therapies. Finally, a review of both the intraoperative and postoperative echocardiographic challenges associated with hypertrophic cardiomyopathy will be presented.
DIAGNOSIS OF AND CLASSIFICATION SYSTEMS FOR HYPERTROPHIC CARDIOMYOPATHY
The diagnosis of hypertrophic cardiomyopathy is made when one detects increased myocardial wall thickness that is not caused by another abnormality, such as hypertension or aortic valve stenosis. The most common manifestation of hypertrophic cardiomyopathy is an asymmetrically thickened ventricular septum (Fig. 22.1). This basal septal thickening narrows the left ventricular outflow tract. Accelerated blood flow within the narrowed left ventricular outflow tract creates a Venturi effect, drawing the mitral valve leaflets and chordal support forward into the subaortic area. This systolic anterior mitral valve motion has become known as “SAM.” This combination of abnormalities leads to one of the hallmarks of this disorder—dynamic left ventricular outflow tract obstruction (Figs. 22.2 and 22.3). The systolic distortion of the mitral valve leaflets can cause significant mitral regurgitation (see Figs. 22.2 and 22.3), increasing the degree of symptoms experienced by the patient.
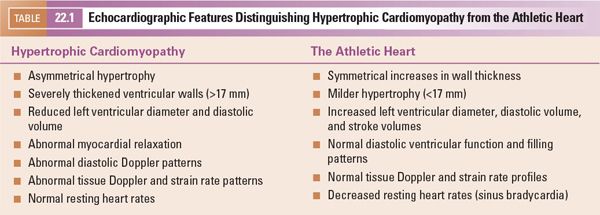
Echocardiography plays a major role not only in diagnosing this disorder but also in excluding secondary causes of hypertrophy. Diagnoses such as coarctation of the aorta and congenital aortic/subaortic stenosis are readily apparent during routine echocardiographic examinations. In older patients, after years of intense athletic training, two-dimensional echocardiographic findings similar to hypertrophic cardiomyopathy may develop. Features that distinguish the athletic heart from hypertrophic cardiomyopathy are summarized in Table 22.1. Clinical tests other than echocardiography are needed to identify patients with systemic hypertension, pheochromocytoma, renal artery stenosis, and/or other forms of renal disease. Diagnoses that are more difficult to exclude on echocardiographic criteria alone include metabolic and storage diseases (Fig. 22.4). The increases in wall thickness associated with these disorders are not caused by true myocyte hypertrophy but rather are caused by storage of abnormal molecules within the myocardial cell. These disorders require clinical correlation and appropriate metabolic screening for accurate identification.
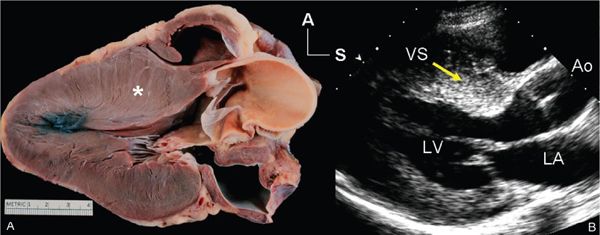
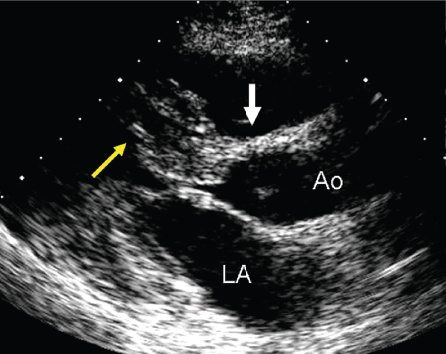
Figure 22.1. Long-axis anatomy typical of hypertrophic cardiomyopathy. A: Anatomic specimen shows a tremendous increase in left ventricular wall thickness, but the increase in myocardial mass is most prominently displayed in the ventricular septum (asterisk). B: Echocardiographic image displays similar anatomy. The increase in myocardial thickness is less prominent than in the anatomic example, but the basal septum (yellow arrow) is still asymmetrically thickened relative to the posterior left ventricular wall. A, anterior; Ao, aorta; LA, left atrium; LV, left ventricle; S, superior; VS, ventricular septum.
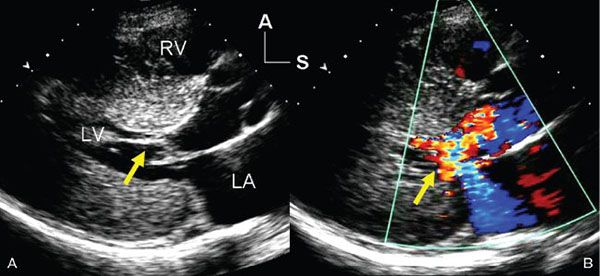
Figure 22.2. Systolic, parasternal long-axis frames demonstrating the left ventricular inflow and outflow tracts of a patient with hypertrophic cardiomyopathy. A: Two-dimensional image shows systolic anterior motion (SAM) of the mitral valve chordal supports (yellow arrow). There was prolonged contact between the chords and the septum beginning in mid-systole and extending through the remainder of ventricular ejection. B: Color flow Doppler image shows not only turbulent flow in the left ventricular outflow tract (as a result of dynamic obstruction), but also an eccentric, posteriorly directed jet of mitral regurgitation (yellow arrow). The dysfunction of the mitral valve is related to the distortion of the leaflets caused by the SAM seen on the left. A, anterior; LA, left atrium; LV, left ventricle; RV, right ventricle; S, superior.
Patients presenting with normal contractility and increased left ventricular wall thickness, analysis of myocardial deformation can be very helpful. Those with hypertrophic cardiomyopathy will generally display a reduction in systolic longitudinal fiber shortening (“less negative” longitudinal strain values) in the regions of most prominent myocardial thickening (see Chapter 4 and Fig. 22.5). In contrast, the patient with secondary hypertrophy and normal systolic function due to other disorders or athletic training will demonstrate normal strain values, even in the thickened segments. It is thought that the myofiber disarray associated with hypertrophic cardiomyopathy is responsible for this deviation from the normal pattern of deformation.
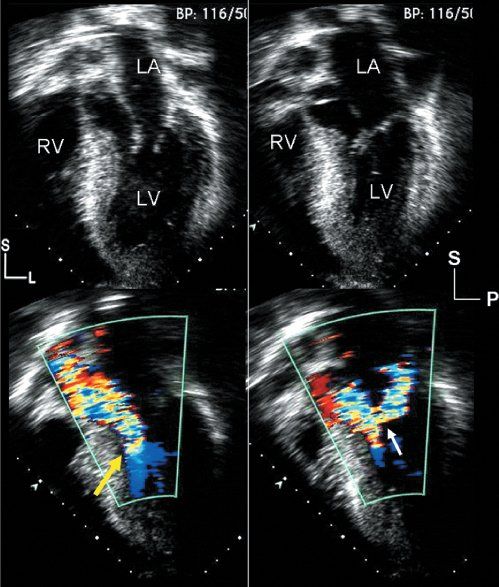
Figure 22.3. Hypertrophic cardiomyopathy: images from the cardiac apex. Left: Images are oriented in a four-chamber format, but the plane of sound has been angled anteriorly, allowing visualization of the left ventricular outflow tract. Right: Images are displayed in an apical, long-axis format, also demonstrating the left ventricular inflow and outflow tracts. Top left: Diastolic frame, which demonstrates the asymmetric, basal ventricular septal thickening that is common with hypertrophic cardiomyopathy. Top right: Systolic frame, which demonstrates the systolic anterior motion of the mitral apparatus, as well as septal contact indicating significant obstruction. Bottom: Two color flow Doppler images demonstrate the primary physiologic consequences of this disorder. The early systolic frame (bottom left) shows narrowing of the left ventricular outflow tract between the hypertrophied septum and the deviated mitral apparatus (yellow arrow). The color flow signal displays turbulence and aliasing at this level, indicating outflow tract obstruction extending at least to this level. The image on the bottom right was taken later in systole. The distortion of the mitral valve associated with systolic anterior motion results in inadequate coaptation and late systolic regurgitation. This series of events has been referred to as the “eject, obstruct, leak” phenomenon and can be seen in virtually all patients with the obstructive form of hypertrophic cardiomyopathy. L, left; LA, left atrium; LV, left ventricle; P, posterior; RV, right ventricle; S, superior.
In the normal heart, the ventricular septum and posterior left ventricular wall have similar thicknesses. The normal ventricular septum has a concave curvature, relative to the left ventricular cavity. This curvature is altered in the presence of hypertrophic cardiomyopathy. Patients with hypertrophic cardiomyopathy have been classified based on either this curvature (Fig. 22.6) or the distribution of left ventricular hypertrophy (Fig. 22.7). Both systems are valid. The type of curvature present seems to be related to the presence of an identifiable genetic mutation. Knowing the pattern of hypertrophy present identifies patients who are likely to have or develop obstruction and the surgical approach that may be most beneficial. Classic asymmetric septal hypertrophy (both basal and diffuse), and mid-ventricular hypertrophy usually show reversed septal curvatures and dynamic outflow obstruction, which can be relieved by transaortic myectomy and myotomy. Some patients with sigmoid curves also have typical dynamic outflow obstruction and are effectively treated by surgical interventions. The most severe subset of patients with reversed septal curvatures develops biventricular outflow obstruction. Those with significant biventricular outflow gradients face the greatest risk for sudden, unexpected mortality.
Cases with diffuse, concentric hypertrophy, or hypertrophy that is isolated to the ventricular free wall, usually have a neutral curve and do not manifest obstruction. These morphologies tend to present with diastolic dysfunction and symptoms suggestive of pulmonary venous congestion. As a result, surgical therapy offers little benefit, and medical therapy is the mainstay of treatment for these patients.
Patients with apical hypertrophy have a variable septal curvature, manifest intracavitary gradients, but not develop true outflow tract obstruction. Diastolic dysfunction is often their primary clinical problem. When the severity of hypertrophy compromises the size of the “functional” left ventricular cavity, apical myectomy designed to increase cavity size (and therefore diastolic filling) can reduce symptoms in this group.
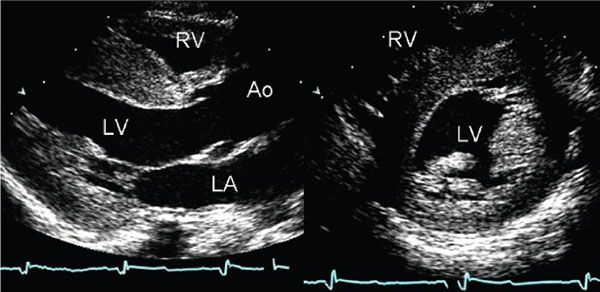
Figure 22.4. Parasternal long- and short-axis images of the left ventricle demonstrate diffuse thickening of the myocardium. The examination was performed in a neonate with no family history of cardiomyopathy. The appearance of these images is similar to the diffuse, nonobstructive form of hypertrophic cardiomyopathy. However, the increased thickness of the myocardium is not caused by hypertrophy in this case, but rather it is secondary to accumulation of glycogen within the myocardial cells. This child was found to have type II glycogen storage disease (Pompe disease). This type of case serves to remind one that echocardiography detects increased wall thickness rather than true hypertrophy and that a diagnosis of hypertrophic cardiomyopathy can only be made after other causes of increased myocardial wall thickness have been excluded. Ao, aorta; LA, left atrium; LV, left ventricle; RV, right ventricle.
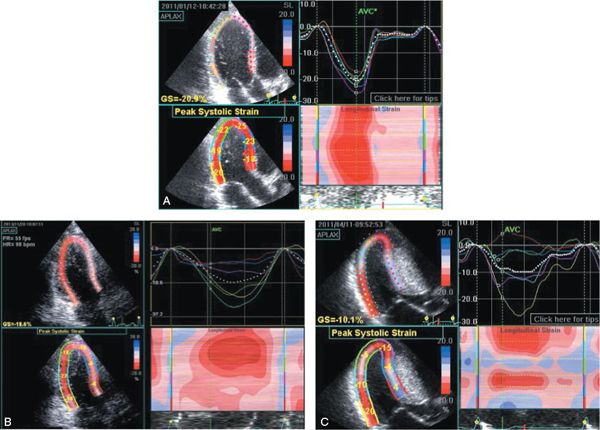
Figure 22.5. These three panels display apical long-axis myocardial strain curves from three different teenagers with increased left ventricular mass. A was taken during an examination of a 17-year-old student athlete, who was referred for sports participation approval. Although his wall thicknesses were greater than expected, the deformation pattern observed in all myocardial segments was normal. This is consistent with athletic training (not cardiomyopathy) being responsible for the increase in mass. B was taken during an examination of a 14-year-old during screening due to a family history of hypertrophic cardiomyopathy. Although she had no outflow obstruction, her septal deformation or strain (blue, red, and lavender curves) is significantly reduced. This implies an abnormality of the myocardium and is consistent with a diagnosis of nonobstructive hypertrophic cardiomyopathy. She was subsequently found to carry the same mutation associated with hypertrophic cardiomyopathy as her relatives. C was taken during an examination of a 15-year-old boy with severe hypertrophic cardiomyopathy in preparation for septal myectomy and defibrillator placement. Note that in his case, the pattern of basal septal deformation is actually “paradoxical.” In other words, the septum immediately adjacent to the aortic annulus (red curve) is actually lengthening (positive strain value) during systole. Only the inferior segment (adjacent to the mitral annulus) shows a normal strain pattern (yellow curve). All of the other segments show the reduced deformation (shortening) that is associated with hypertrophic cardiomyopathy.
ECHOCARDIOGRAPHIC IMAGING IN HYPERTROPHIC CARDIOMYOPATHY
A complete surface echocardiographic evaluation of the patient with hypertrophic cardiomyopathy should include a description of (a) the pattern and severity of myocardial thickening present, (b) the presence and nature of left and/or right ventricular outflow obstruction, (c) the nature of the mitral valve distortion and severity of the resulting regurgitation, (d) the size of the left atrium (shown to be associated with the clinical disease burden), (e) left ventricular diastolic filling, and (f) the pulmonary arterial pressure.
The typical anatomy of obstructive hypertrophic cardiomyopathy is often most easily recognized in the parasternal long-axis views. There is often a prominent basal septal bulge present and the distortion of the mitral support structures can be easily appreciated (see Figs. 22.1 and 22.2). Apical four-chamber and long-axis views can reveal the same findings (see Fig. 22.3) and are better suited for some Doppler echocardiographic interrogations. The magnitude of septal thickening has been associated with the incidence of sudden death. Patients with diastolic septal thicknesses greater than 30 mm have an increased risk of sudden death events, while such events rarely occur if the septal diameter is less than 19 mm. This thickness is best assessed using parasternal two-dimensional scans (Fig. 22.1). These scans allow the examiner to determine the point of maximal diastolic thickness and to avoid inclusion of the right ventricular papillary muscles in the measurement. Systolic ventricular contraction is usually normal or hyperdynamic in this disorder. Systolic ventricular dysfunction is only seen in the most advanced stages of this disease, after years of obstruction and progressive myocardial fibrosis. In contrast, diastolic dysfunction is present in nearly all patients with hypertrophic cardiomyopathy. The associated elevations in left atrial and ventricular diastolic pressures contribute significantly to patients’ symptoms.
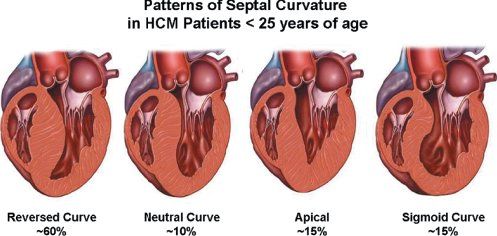
Figure 22.6. Variety of septal curvatures that can exist in patients with hypertrophic cardiomyopathy. By far the most common morphology seen in childhood is the reversed septal curve. Any of these patterns can be associated with outflow obstruction. Diastolic dysfunction also occurs in all of these groups but tends to be most severe in those with neutral septal curves or the apical variant of hypertrophic cardiomyopathy (HCM).
Parasternal long-axis views also provide a unique insight into surgical planning. When obstruction is present, the examiner should define the distance between the anatomic aortic valve annulus and the point of contact between the mitral apparatus and the ventricular septum that is farthest from the aortic valve. This distance represents the minimum extent to which a septal myectomy should be performed (Fig. 22.8). Early surgical therapy, first proposed by Morrow, created a single trough in the hypertrophied ventricular septum, allowing an unobstructed channel for ejection (Fig. 22.9). In many patients, the obstructive zone involves the papillary muscles themselves. In these cases, an extended septal myectomy is required (Fig. 22.10) to provide adequate enlargement of the outflow tract. In patients with a small aortic annulus or obstructions extending deep into the ventricle (beyond the mid-papillary muscle level), a combination of transaortic and apical myectomies may be necessary (Fig. 22.11).
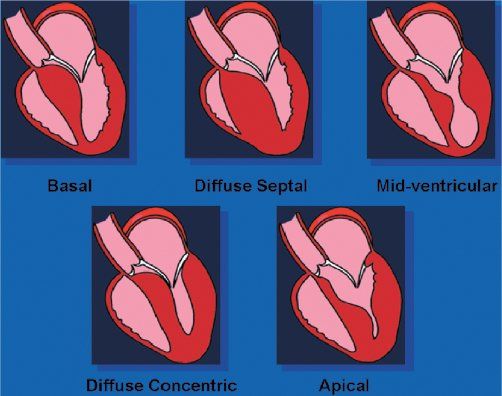
Figure 22.7. Shape of the ventricular septum in patients with hypertrophic cardiomyopathy. Top: Patients with these septal geometries often develop significant, dynamic outflow obstruction. The obstruction seen in these three morphologies will usually be effectively treated with transaortic, extended septal myectomy. Bottom: Combined transaortic and transapical approaches may be necessary when the hypertrophy is more diffuse. In the rare patient in whom apical hypertrophy limits ventricular diastolic filling, transapical myectomy may increase the diastolic filling potential.
The diffuse, nonobstructive, and apical form of hypertrophic cardiomyopathy requires multiple planes of imaging for confident recognition. The ventricular walls are concentrically thickened. No accelerated color flow Doppler signals will be seen during systole, and the mitral apparatus will move normally. The prominent diastolic dysfunction associated with this type of myopathy will usually cause significant left atrial enlargement.
The thickened segments of myocardium in patients with apical hypertrophic cardiomyopathy are often not visible from the parasternal window (Fig. 22.12). Apical four-chamber and long-axis scans will demonstrate obliteration of the apical portion of the left ventricular cavity by hypertrophied muscle (Fig. 22.13). High-velocity flow signals can be found in the left ventricular apex. These systolic flow accelerations are directed toward the mid-ventricular cavity, but the true outflow tract is usually widely patent. The potential left ventricular diastolic volume is often reduced in these patients, further compounding the diastolic dysfunction associated with this form of cardiomyopathy. In select cases, transapical resection of the inner myocardial segments may be helpful, increasing the potential diastolic ventricular stroke volume in these patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


