Hypertrophic Cardiomyopathy
Paul Sorajja MD, FSCAI
Hypertrophic cardiomyopathy (HCM) is a common, inheritable cardiac disorder with a prevalence of 1 in 500 persons. HCM is defined as the presence of severe myocardial hypertrophy in the absence of a known local or systemic etiology (1). In 1989, molecular studies first demonstrated that HCM is due to mutations in sarcomeric genes. Over 200 mutations in 10 different genes have since been identified in patients with this disorder.
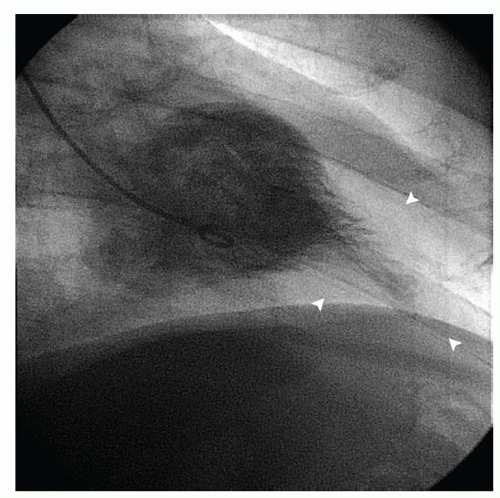
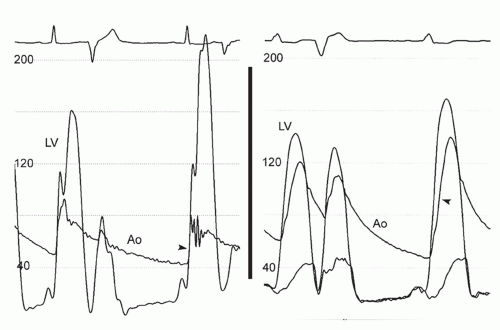
The clinical diagnosis of HCM is typically made by detection of severe myocardial hypertrophy using two-dimensional echocardiography and, in some cases, cardiac magnetic resonance imaging (MRI). Doppler echocardiography can accurately diagnose and quantify left ventricular outflow tract (LVOT) obstruction using the modified Bernoulli equation (gradient = 4 velocity2) in most instances. During cardiac catheterization, the operator should suspect HCM when there is a hypertrophied left ventricle with a small cavity and normal or hyperdynamic systolic function on ventriculography. Regional hypertrophy, such as basal septal or apical hypertrophy, may be present (Fig. 42-1). Left ventricular diastolic pressures may be elevated, reflecting diastolic dysfunction. Dynamic LVOT obstruction should be suspected if there is a gradient between the left ventricular apex and base or if there is a “spike and dome” pattern on the aortic pressure trace (Fig. 42-2). Of note, the presence of dynamic LVOT obstruction is highly dependent on ventricular loading conditions and the contractile state, and may be evident only with physical maneuvers or drug provocation.
PATHOPHYSIOLOGY
Diastolic dysfunction is the major pathophysiological mechanism contributing to signs and symptoms for patients with HCM. Abnormalities of diastolic dysfunction arise due to abnormal relaxation and poor compliance in the presence of altered loading conditions, ventricular non-uniformity, severe hypertrophy, and myocardial ischemia. The end result of diastolic dysfunction is an increase in left ventricular filling pressures that causes the typical symptoms of dyspnea and angina. For patients with HCM, there may be a significant discrepancy between the mean left atrial pressure and left ventricular end diastolic pressure. Therefore, both measurements should be made when possible.
In up to two-thirds of HCM patients, a dynamic LVOT gradient may be present (2). Documentation of the presence and severity of the LVOT gradient is essential as this then provides the basis for therapy. Two mechanisms underlie the development of dynamic LVOT obstruction: (a) septal hypertrophy and narrowing of the LVOT, which lead to Venturi forces that accelerate during ventricular emptying and pull the mitral apparatus anteriorly; and (b) anterior papillary muscle displacement, which subjects the mitral leaflets to intraventricular currents during systole that drag the apparatus anteriorly. Decreased mitral leaflet coaptation occurs due to systolic anterior motion of the mitral valve, leading to secondary mitral regurgitation in patients with LVOT obstruction.
It is important to note the dynamic nature of LVOT obstruction and secondary mitral regurgitation. LVOT obstruction is
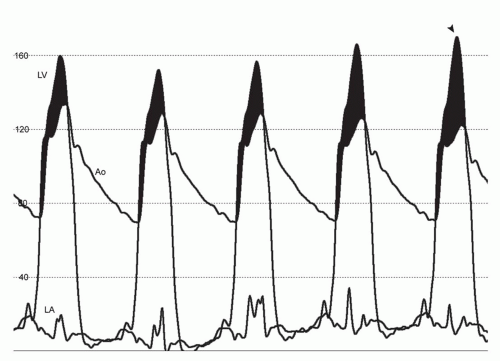
exacerbated by increases in inotropy and decreases in either ventricular afterload (e.g., vasodilators) or preload (e.g., dehydration, diuretic therapy). The severity of LVOT obstruction is highly sensitive to ventricular load and contractility, with changes in the gradient even observed during quiet respiration (Fig. 42-3). Although the clinical significance of LVOT obstruction in HCM has been debated, recent studies have linked its presence to heightened risk for heart failure and, in some reports, an increased risk of death.
exacerbated by increases in inotropy and decreases in either ventricular afterload (e.g., vasodilators) or preload (e.g., dehydration, diuretic therapy). The severity of LVOT obstruction is highly sensitive to ventricular load and contractility, with changes in the gradient even observed during quiet respiration (Fig. 42-3). Although the clinical significance of LVOT obstruction in HCM has been debated, recent studies have linked its presence to heightened risk for heart failure and, in some reports, an increased risk of death.
MEDICAL THERAPY
Negative inotropic agents, such as β-receptor antagonists, disopyramide, and calcium-channel blockers (i.e., verapamil, diltiazem), are the cornerstone of drug therapy for symptomatic LVOT obstruction. By depressing contractility, these agents increase diastolic filling time, improve myocardial relaxation, reduce the imbalance of myocardial oxygen supply and demand, and ameliorate the propensity toward LVOT obstruction by reducing the intraventricular flow velocities that aggravate the development of systolic anterior motion of the mitral valve. It is important to note that large doses of these medications are frequently required (e.g., 480 mg verapamil or 400 mg propanolol). Verapamil should be used only with caution, if at all, in patients with advanced heart failure, high LVOT gradients, and bradycardia. Furthermore, disopyramide should be prescribed with a concomitant atrioventricular (AV) node blocker in patients with atrial fibrillation, as disopyramide may accelerate AV conduction.
In addition, peripheral vasodilators, inotropic agents (e.g., digoxin, β-receptor agonists), and high-dose diuretics should be avoided as they will aggravate the development of LVOT obstruction. Patients should also be counseled on the need to maintain hydration and general avoidance of circumstances that precipitate vasodilatation (e.g., saunas). When severe symptoms persist despite optimal drug therapy, definitive septal reduction therapy should be considered in patients with obstructive HCM.
SURGICAL MYECTOMY
The time-honored standard for septal reduction therapy is surgical myectomy (3, 4 and 5). In this procedure, a surgeon uses a transaortic approach to widen the LVOT through direct resection of the hypertrophied ventricular septum. Not uncommonly, the myectomy is extended to the base of the papillary muscles, leading to full reconstruction of the LVOT. While early historical series raised concern about the safety of the procedure, surgical myectomy now has an operative mortality of <1% with a success rate of >90% when performed in experienced centers. Complications, such as aortic regurgitation, ventricular septal defect, and pacemaker dependency are infrequent (<5%). Importantly, long-term studies (≥ 0 year follow-up) have demonstrated no impairment of survival after surgical myectomy. In current national practice guidelines on HCM, surgical myectomy is the preferred mode of therapy for septal reduction in highly symptomatic patients (1).
ALCOHOL SEPTAL ABLATION
In 1995, percutaneous alcohol septal ablation was introduced as an alternative to surgical myectomy for the relief of LVOT obstruction in patients with HCM. The aim of alcohol septal ablation is to induce a localized myocardial infarction and thinning of the basal ventricular septum, thereby leading to a reduction in septal thickening and systolic excursion into the LVOT.
Patient Selection
Proper patient selection is critical to the success of septal ablation. A comprehensive clinical evaluation and echocardiogram should be performed in all candidates, ideally in a center with expertise in the care of HCM patients. Criteria for septal ablation include: (a) severe, drug-refractory cardiac symptoms (New York Heart Association functional class III/IV dyspnea or Canadian Cardiac Society angina class III/IV) due to obstructive HCM; (b) dynamic LVOT obstruction (gradient ≥30 mm Hg at rest or ≥50 mm Hg with provocation) that is due to septal hypertrophy and systolic anterior motion of the mitral valve; (c) ventricular septal thickness ≥15 mm; (d) absence of significant intrinsic mitral valve disease; (e) absence of need for concomitant cardiac surgical procedure (e.g., bypass grafting, valve replacement); and (f) informed patient consent. A comprehensive two-dimensional and Doppler echocardiogram is elementary to proper patient selection. This evaluation should document the dynamic nature of the LVOT obstruction and exclude anatomic findings that would impede the clinical efficacy of the procedure (Figs. 42-4 and 42-5).
Informed patient consent requires full understanding of the limited data on long-term survival after the procedure, risk of pacemaker dependency, the relatively lower success rate due to its dependence on coronary anatomy, and potential complications related to cardiac catheterization and instrumentation of the coronary arteries. It is advised that decision making regarding surgical myectomy or alcohol septal ablation be undertaken in the context of a longitudinal, multidisciplinary program with expertise in the care of HCM patients, with experienced operators performing these procedures (1).
Although younger age is not an absolute contraindication to the procedure, septal ablation has generally been reserved for older adult patients due to the limited data on long-term survival of the procedure. In the 2011 ACCF/AHA practice guidelines on HCM, septal ablation is a class III recommendation for patients under the
age of 21 years, and the procedure is also strongly discouraged in those aged <40 years if surgical myectomy is a viable option (1).
age of 21 years, and the procedure is also strongly discouraged in those aged <40 years if surgical myectomy is a viable option (1).
Hemodynamic Evaluation
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree