■ Two hybrid approaches are described in this chapter.
■ Complete visceral debranching and endovascular tube graft repair
■ Partial visceral debranching and physician-modified fenestrated endovascular repair
DIFFERENTIAL DIAGNOSIS
■ Paravisceral aortic aneurysms may develop due to the following conditions:
■ Degenerative aneurysm
■ Aortic dissection
■ Mycotic aneurysm
■ Paraanastomotic juxtarenal aneurysm
■ Connective tissue disorders (Marfan’s syndrome)
■ Behçet syndrome
PATIENT HISTORY AND PHYSICAL FINDINGS
■ The majority of patients are asymptomatic and the diagnosis is made with imaging done for other reasons. Some patients will complain of mild to moderate abdominal and low back pain. Severe and unrelenting pain should raise the index of suspicion for a mycotic process which, if confirmed, would make hybrid approaches prohibitive.
IMAGING AND OTHER DIAGNOSTIC STUDIES
■ Contrast-enhanced, axial thin-slice computed tomography arteriography (CTA) is the current standard for imaging paravisceral aneurysms. Detailed information can be gathered regarding the precise origin of the celiac, superior mesenteric artery (SMA), and renal arteries (FIG 2).
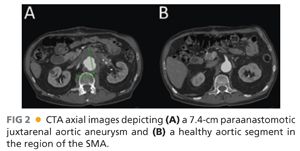
■ Other important findings on CTA should be as follows:
■ Size and quality of access vessels for delivery of endovascular devices (>7 mm)
■ Location of left renal vein
■ Aberrant anatomy (e.g., replaced right hepatic artery)
■ Quality of gastroduodenal artery for possible celiac artery ligation or sacrifice
■ Renal cortical thickness
SURGICAL MANAGEMENT
■ Indications for repair include aortic aneurysms of more than 5.5 cm, symptoms, or evidence of rapid expansion (>0.5 cm per 6 months).
Preoperative Planning
■ As formal open repair would often include a bicavitary incision (chest and abdomen, as in a formal thoracoabdominal repair), the standard preoperative assessment should focus on the patient’s fitness to undergo major vascular surgery. This includes assessment of heart, lung, and kidney function and reserve.
Positioning
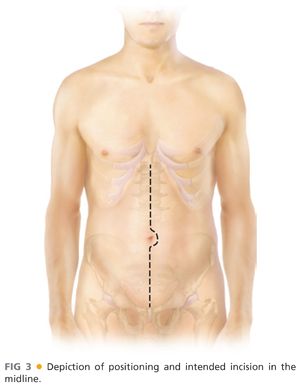
■ Proper and precise positioning should be as follows (FIG 3):
■ Patient supine on standard operating room table or imaging table
■ Hair properly clipped over entire abdomen and both groins
■ Both arms tucked (option to have right arm at 90 degrees if planning brachial access)
■ Foley under one leg and padded
TECHNIQUES
COMPLETE VISCERAL DEBRANCHING AND ENDOVASCULAR TUBE GRAFT REPAIR—STAGE 1
First Step—Exposure
■ Standard midline laparotomy and positioning of retractor system
■ Upon entry into the abdomen, the falciform ligament is divided between clamps and ligated. The triangular ligaments above the liver are divided to facilitate adequate exposure/retraction while minimizing risk of hepatic capsular injury, anticipating systemic anticoagulation later in the procedure.
■ A nasogastric tube is positioned in the stomach to provide temporary decompression. The common hepatic artery is identified following division of the gastrohepatic ligament and traced back to origin of celiac artery. Once identified, the target artery is encircled with a silastic vessel loop. Space is created along the left side of the aorta with blunt/finger dissection, beginning at the level of the celiac artery, to create the retrograde bypass tunnel posterior to the pancreas (FIG 4).

■ The colon and omentum are lifted in a cephalad direction, the small bowel swept to the patient’s right and packed in moist towels. Self-retaining retractors (Omni or Bookwalter) should be positioned at this juncture to maintain exposure, with care taken to appropriately pad the retractor blades as necessary.
■ The third and fourth portions of the duodenum are mobilized to the right following division of the ligament of Treitz, exposing the anterior surface of the aorta. The inferior mesenteric vein is ligated and divided as well and the dissection continued along the proximal aorta until the left renal vein is clearly identified (FIG 5).
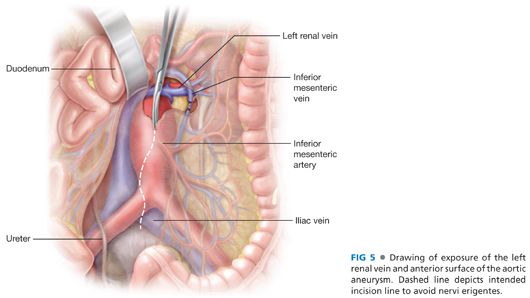
■ Widely mobilize the left renal vein sharply and encircle with a moist umbilical tape. The self-retaining renal vein retractor blade is used to retract the left renal vein cephalad as necessary to facilitate further exposure.
■ The origin of the renal arteries is identified by careful posterolateral dissection around the aorta, just cephalad of the overlying renal vein. Exposure on the right is complicated somewhat by the overlying inferior vena cava/left renal vein confluence. At least 2 cm of renal artery should be exposed bilaterally. Encircle the renal arteries with silastic vessel loops. On the left, finger dissect bluntly along the aorta in a cephalad fashion to complete the retropancreatic tunnel for the celiac limb of the bypass graft.
■ The SMA is identified next by palpation within the base of the small bowel mesentery, directly anterior to the pancreas. Doppler ultrasonography may assist identification when the pulse is faint. Once identified, a 3-cm segment of SMA is isolated as proximal as possible to the root of the mesentery. Beginning with the middle colic artery, multiple mesenteric arteries quickly branch from the SMA as it emerges from the pancreas, underscoring the need for proximal identification and isolation. The SMA is controlled with vessel loops.
■ The next step is to prepare the donor artery for hybrid bypass. The specific artery—most commonly the common or external iliac arteries—should be selected from the preoperative imaging study. The retroperitoneum is opened directly over the selected donor artery, which is exposed while protecting the adjacent ureter. Alternatively, donor artery exposure may be achieved via medial-visceral rotation, developing the entire retroperitoneal plane on the left. The latter approach provides the added benefit of exclusion of the graft from the viscera and abdominal contents once the viscera are returned to their original position. This maneuver adds significantly more time to the case, however, and contributes to increased blood loss. Graft coverage can also be obtained without developing the entire retroperitoneal plane, either via direct tunneling along the preferred course of the graft or creation of an omental tongue affixed directly to the graft.
Second Step—Anticoagulation
■ Systemic anticoagulation is achieved with a bolus injection of unfractionated heparin, 50 units/kg. Monitoring activated clotting time is a useful method of maintaining adequate anticoagulation during the procedure.
Third Step—Multivisceral Bypass
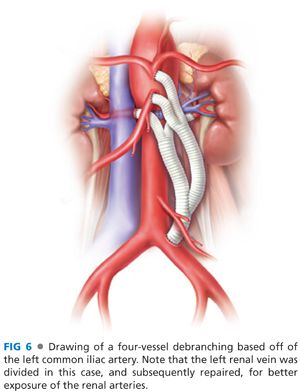
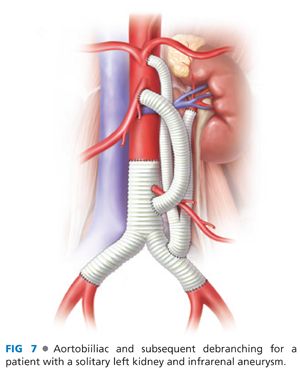
■ Trifurcated grafts exist for the purpose of facilitating multivessel hybrid revascularization, but the use of these are limited by the tendency of the middle limb to occlude when “squeezed” between the outside limbs during graft routing and abdominal closure. In most circumstances, a standard 12 × 7 bifurcated, collagen-impregnated knitted polyester graft provides excellent conduits for bilateral renal revascularization, with a separate 8-mm limb connected to the celiac and SMA. Examples of bypass graft configurations are shown in FIGS 6 and 7.
■ The proximal (iliac/inflow) anastomosis is completed first with running 4–0 or 5-0 polypropylene suture.
■ The next anastomosis to be completed should be one anticipated to be the technically most difficult, given exposure and graft routing issues. Most commonly, this is the right renal artery. This is divided following placement of a large clip at the origin. The appropriate graft limb is pulled to length and anastomosed end-to-end with 5-0 polypropylene suture. The limb and artery are flushed just prior to completion of the graft, after which the clamps are released to reperfuse the kidney. Following this sequence, warm renal ischemia time is generally less than 12 minutes. The stump of the right renal artery is then suture ligated; avoid clip dislodgement. Note: Excessive traction on the confluence of the left renal vein and vena cava may cause caval injury and massive hemorrhage during preparation and completion of the right renal artery anastomosis. Retractor positioning needs to account for potential venous injury during exposure and significantly relaxed following completion of the anastomosis.
■ The left renal anastomosis is completed in nearly identical fashion, minus many of the exposure limitations present on the right.
■ The SMA graft is carefully sized to length so that it follows a “C”-shaped configuration without kinking. Inflow can be obtained either from the many bodies of the graft or either of the completed renal limbs. The SMA-graft anastomosis is completed end-to-side with interrupted or running 5-0 polypropylene suture. The end-to-side arteriotomy length is 1.5 to 2 times the width of the bypass graft (12 to 16 mm). Alternatively, end-to-end anastomotic configuration may reduce the likelihood of graft kinking depending on final configuration. Following completion of the anastomosis, the proximal SMA is ligated with a large clip or circumference suture. Again, ischemia time should be under 10 to 12 minutes.
■ Typically, following SMA and renal graft completion, repositioning of the retraction system is necessary to reobtain and optimize celiac artery exposure. Prior to reexposing the celiac, a vascular clamp is repassed through the retropancreatic tunnel left of the aorta. This position is then maintained until the transverse colon and mesocolon are reduced to their usual location. This reexposes the “looped” celiac and common hepatic arteries previously isolated in the lesser sac. The clamp tip exiting the retrohepatic tunnel is identified, and a moist umbilical tape is pulled through the tunnel. Following this, the celiac limb is tied to the umbilical tape, which is then pulled cephalad behind the pancreas and into position for either end-to-end or end-to-side anastomosis. Care again needs to be taken to optimize limb routing and length to minimize risk for kinking.
■ After coverage of remaining exposed graft limbs with omentum or parietal peritoneum as appropriate, standard abdominal closure is performed.
COMPLETE VISCERAL DEBRANCHING AND ENDOVASCULAR TUBE GRAFT REPAIR—STAGE 2
First Step—Percutaneous Access
■ Following the “debranching” procedure described in stage 1, endovascular aneurysm repair (EVAR) may be performed either at the same setting or within several weeks of the initial procedure. The risk of potential aneurysm rupture associated with a staged approach needs to be balanced with the additional operative risk inherent in the longer anesthetic time required to complete both stages in one sitting. For the EVAR procedure itself, standard percutaneous access to an appropriately sized access vessel is obtained using Seldinger technique and a wire advanced into the aorta under fluoroscopic guidance. In our practice, this is most commonly obtained percutaneously, using ultrasound guidance and preplacement of polypropylene suture prior to dilation of the access sites (also known as the “preclose” Perclose® technique (Abbott Vascular Inc, Redwood City, CA).1 An 11-Fr standard sheath is placed into the common femoral artery and flushed with heparinized saline. Wire advancement from the femoral artery to the aortic arch must be visualized radiographically throughout its course, as the wire may preferentially enter the debranching graft and cause end-organ injury or hemorrhage without real-time position monitoring and guidance.
Second Step—Stiff Wire Exchange
■ After wire advancement to the transverse aortic arch, standard wire exchange technique is used to position a 0.035-in stiff (e.g., Lunderquist®, Cook Medical, Bloomington, IN) wire through the abdominal and thoracic aorta. Optimal final wire positioning is at/just distal to the left subclavian artery orifice.
Third Step—Intravascular Ultrasound
■ An 8.2-Fr Visions® catheter (Volcano Therapeutics, Irvine, CA) is used to confirm appropriate proximal and distal landing zones for endovascular graft placement. The optimal graft size and configuration is determined by analysis of CTA images reformatted and visualized on a dedicated 3-D image workstation (AquariusNet®, TeraRecon, Inc, San Mateo, CA). Graft diameter should be oversized by 10% to 15% for this application.
■ During advancement of the device, the origin of the debranching graft can also be visualized either through fluoroscopic confirmation of a metallic clip placed during the debranching procedure or under intravascular ultrasound (IVUS) real-time guidance. Using IVUS, the position of the IVUS catheter is marked on the fluoroscopic monitor when the catheter itself recognizes the orifice of the debranched graft. Alternatively, a contrast power injection can be performed through an appropriately positioned arteriographic catheter with 30 mL of contrast injected at 15 mL per second to confirm the proximal and distal landing zones.
Fourth Step—Endograft Deployment
■ The endovascular graft is deployed following device-specific instructions for use (IFU), covering the native origins of the visceral vessels and excluding the aortic aneurysm. The femoral arteriotomy is then closed.
PARTIAL VISCERAL DEBRANCHING AND PHYSICIAN-MODIFIED ENDOVASCULAR REPAIR—STAGE 1
First Step—Exposure
■ Standard midline laparotomy and positioning of retractor system.
■ Upon entry into the abdomen, the falciform ligament is divided between clamps and ligated. The triangular ligaments above the liver are divided to facilitate adequate exposure/retraction while minimizing risk of hepatic capsular injury, anticipating systemic anticoagulation later in the procedure.
■ A nasogastric tube is positioned in the stomach to provide temporary decompression. The common hepatic artery is identified following division of the gastrohepatic ligament and traced back to origin of celiac artery. Once identified, the target artery is encircled with a silastic vessel loop. Space is created along the left side of the aorta with blunt/finger dissection, beginning at the level of the celiac artery, to create the retrograde bypass tunnel posterior to the pancreas.
■ The colon and omentum are lifted in a cephalad direction, the small bowel swept to the patient’s right and packed in moist towels. Self-retaining retractors (Omni or Bookwalter) should be positioned at this juncture to maintain exposure, with care taken to appropriately pad the retractor blades as necessary.
■ The third and fourth portions of the duodenum are mobilized to the right following division of the ligament of Treitz, exposing the anterior surface of the aorta. The inferior mesenteric vein is ligated and divided as well and the dissection continued along the proximal aorta until the left renal vein is clearly identified.
■ Widely mobilize the left renal vein sharply and encircle with a moist umbilical tape. The self-retaining renal vein retractor blade is used to retract the left renal vein cephalad as necessary to facilitate further exposure.
■ The origin of the renal arteries is identified by careful posterolateral dissection around the aorta, just cephalad of the overlying renal vein. Exposure on the right is complicated somewhat by the overlying inferior vena cava/left renal vein confluence. At least 2 cm of renal artery should be exposed bilaterally. Encircle the renal arteries with silastic vessel loops. On the left, finger dissect bluntly along the aorta in a cephalad fashion to complete the retropancreatic tunnel for the celiac limb of the bypass graft.
■ The SMA is identified next by palpation within the base of the small bowel mesentery, directly anterior to the pancreas. Doppler ultrasonography may assist identification when the pulse is faint. Once identified, a 3-cm segment of SMA is isolated as proximal as possible to the root of the mesentery. Beginning with the middle colic artery, multiple mesenteric arteries quickly branch from the SMA as it emerges from the pancreas, underscoring the need for proximal identification and isolation. The SMA is controlled with vessel loops.
■ The next step is to prepare the donor artery for hybrid bypass. The specific artery—most commonly the common or external iliac arteries—should be selected from the preoperative imaging study. The retroperitoneum is opened directly over the selected donor artery, which is exposed while protecting the adjacent ureter. Alternatively, donor artery exposure may be achieved via medial-visceral rotation, developing the entire retroperitoneal plane on the left. The latter approach provides the added benefit of exclusion of the graft from the viscera and abdominal contents once the viscera are returned to their original position. This maneuver adds significantly more time to the case, however, and contributes to increased blood loss. Graft coverage can also be obtained without developing the entire retroperitoneal plane, either via direct tunneling along the preferred course of the graft or creation of an omental tongue affixed directly to the graft.
Second Step—Anticoagulation
■ Systemic anticoagulation is achieved with a bolus injection of unfractionated heparin, 50 units/kg. Monitoring activated clotting time is a useful method of maintaining adequate anticoagulation during the procedure.
Third Step—Multivisceral Bypass
■ Trifurcated grafts exist for the purpose of facilitating multivessel hybrid revascularization, but the use of these are limited by the tendency of the middle limb to occlude when squeezed between the outside limbs during graft routing and abdominal closure. In most circumstances, a standard 12 × 7 bifurcated, collagen-impregnated knitted polyester graft provides excellent conduits for bilateral renal revascularization, with a separate 8-mm limb connected to the celiac and SMA. Examples of bypass graft configurations are shown in FIGS 6 and 7.
■ The proximal (iliac/inflow) anastomosis is completed first with running 4-0 or 5-0 polypropylene suture.
■ The next anastomosis to be completed should be one anticipated to be the technically most difficult, given exposure and graft routing issues. Most commonly, this is the right renal artery. This is divided following placement of a large clip at the origin. The appropriate graft limb is pulled to length and anastomosed end-to-end with 5-0 polypropylene suture. The limb and artery are flushed just prior to completion of the graft, after which the clamps are released to reperfuse the kidney. Following this sequence, warm renal ischemia time is generally less than 12 minutes. The stump of the right renal artery is then suture ligated; avoid clip dislodgement. Note: Excessive traction on the confluence of the left renal vein and vena cava may cause caval injury and massive hemorrhage during preparation and completion of the right renal artery anastomosis. Retractor positioning needs to account for potential venous injury during exposure and significantly relaxed following completion of the anastomosis.
■ The renal anastomosis is completed in nearly identical fashion, minus many of the exposure limitations present on the right.
PARTIAL VISCERAL DEBRANCHING AND PHYSICIAN-MODIFIED ENDOVASCULAR REPAIR—STAGE 22
First Step—Creation of a Fenestrated Graft for the Celiac and Superior Mesenteric Artery
■ The appropriate endovascular device is chosen according to standard IFU sizing guidelines, typically incorporating 10% to 15% oversizing. The sterile graft is unsheathed on a dedicated sterile table in the operating room and marked with the relative locations (length from proximal end and clockface measurements) of the celiac and SMA fenestrations as previously determined via TeraRecon® workstation analysis. Minor adjustments are allowed to minimize strut overlap of planned fenestration locations. Fenestrations in the polyester endograft fabric are created with a disposable ophthalmic cautery to minimize fraying. The fenestrations are outlined and reinforced with 15-mm gold Amplatz Gooseneck® snares (ev3 Endovascular, Inc, Plymouth, MN). These are hand sewn into place using 4-0 Prolene suture in a double row circumferentially (FIG 8). Diameter-reducing ties were then used to constrain the device along its posterior border (opposite the SMA and or celiac fenestration at 6 o’clock) by rerouting the existing proximal trigger wire through and through the graft material at the midportion of each of the top two Z stents. The constraining ties are then tied down into place over the trigger wire. The entire graft is then wetted with heparinized saline and then reloaded into the existing sheath.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


