Fig. 25.1
John Dilorio (Dr. Iaizzo’s cousin) was a young cardiac patient of Dr. Lillehei and his team, shown here in 1958 (a) in his hospital bed at the University Variety Club Hospital and then (b) leaving the hospital with his father

Fig. 25.2
“The Chief” Owen H. Wangensteen, the youngest Surgery Department Chairman at age 31, served as chairman of the department from 1930 to 1967
Table 25.1
Department of Surgery at the University of Minnesota: chairs/interim heads
Surgery Department chair/interim head | Position | Years served |
|---|---|---|
Arthur C. Strachauer | Department Chair | -1925, 1927–1929 |
Owen H. Wangensteen | Department Chair | 1930–1967 |
John S. Najarian | Department Chair | 1967–1993 |
Edward W. Humphrey | Interim Chair | 1993–1994 |
Frank B. Cerra | Interim Chair | 1994–1995 |
David L. Dunn | Department Chair | 1995–2005 |
David A. Rothenberger | Interim Chair | 2005–2006 |
Selwyn M. Vickers | Department Chair | 2006–2013 |
David A. Rothenberger | Department Chair | 2013–present |
In the early 1950s, the innovative surge was credited to the fact that many surgical residents were returning from World War II, where they had experienced life and death situations when managing surgical field units. They had little or no fear of death and their generation was not afraid to “push the envelope” to help patients. By today’s standards, these residents would be viewed as mavericks but, in fact, they had little to lose, not unlike situations they faced on the battlefield. Their heart patients were dying and/or had little chance of survival without the novel techniques that were successfully implemented in Minnesota.
One of these young war-experienced surgeons was C. Walton Lillehei, who returned to the University of Minnesota in 1950 to complete his surgical residency after leading an army surgical field unit in both North Africa and Italy (Fig. 25.3). Lillehei was very bright (he also completed M.S. and Ph.D. degrees during this time) and was known as an impulsive maverick , always pushing to the next level of care for his clinical patients for whom he had great empathy. Lillehei and his team launched many surgical innovations during this period, primarily due to their hands-on research experience in the experimental dog laboratories; one site for this research was located in the basement of the Mayo Hospital building, just three floors below the main operating rooms. Today, this lab space houses the Visible Heart® Laboratory under the direction of Dr. Paul Iaizzo (Figs. 25.4 and 25.5) [2].

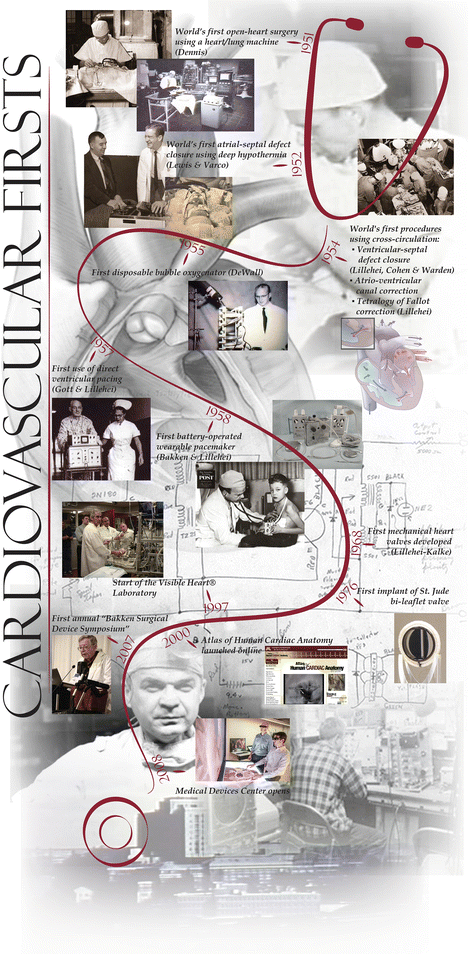

Fig. 25.3
Walt Lillehei in his army uniform

Fig. 25.4
Schematic of “cardiovascular firsts” at the University of Minnesota

Fig. 25.5
Photo of Dr. Paul Iaizzo in the Visible Heart® Laboratory at the University of Minnesota
Interestingly, prior to 1950, the heart was considered to be the core of human emotion, even the soul itself. For perspective, Table 25.2 depicts some highlights of a typical operating room in the 1950s. Relative to open-heart surgery in 1951, congenital heart defects were responsible for 1 % of all deaths in this age group; thus the prognosis was poor for a child with such a defect. There were no methods for conducting external heart surgery and no way to oxygenate the brain during surgery. In other words, attempts were made to repair a patient’s heart while it remained beating, obscuring the view with blood; any stoppage of blood flow would result in damage of the brain. Therefore, only the simplest surgeries could be performed on the beating heart.
Table 25.2
1950s operating room environment
• No computers or digital equipment existed |
• Glass thermometers, blood pressure cuffs, and a finger pressed against the wrist were used to obtain vital signs |
• Anesthesia was supplied on a soaked rag and delivered by squeezing the black bag |
• (Nonoperating room) X-rays were the only imaging equipment available |
• Flammable gases could cause explosions in the operating room |
• Head lamps lit the operative field |
• The second hand on a wall clock was one of the most useful instruments to a surgeon |
• No pulse oximeters, pacemakers, blood-gas analyzers, or specialized imaging existed |
When the medical profession eventually began to view the heart more physiologically, as a pump or machine within the body, researchers and clinicians began to develop new ways to repair and replace worn-out parts of the heart. Innovations in the field of cardiac surgery then flourished (Table 25.3). Such innovation became prominent at the University of Minnesota. For example, Dr. Clarence Dennis designed one of the first heart–lung machines for total cardiopulmonary bypass, which was subsequently tested successfully on dogs (Figs. 25.4 and 25.6). However, when Dennis and his team used the heart–lung machine in the clinical area for the first time on April 5, 1951, the patient died due to complications; a second patient also died during surgery from a massive air embolism. Not long after, Dr. Dennis moved his machine and most of his team to New York City [1].

Table 25.3
University of Minnesota milestones
1887 | New standards requiring medical students to pass exams and gain medical examining board approval (led by Medical School Dean, Perry Millard) |
1911 | Minnesota became the first state to mandate hospital internships for medical students |
1930s | Discovery of the link between cholesterol and heart disease (Ancel Keys) |
1950 | First adaptation of the mass spectrograph (Alfred Nier) |
1951 | First attempt to use a heart–lung machine (Clarence Dennis) |
1952 | First successful open-heart surgery using hypothermia (F. John Lewis) |
1953 | First jejunoileal bypass (Richard L. Varco) |
1954 | First open-heart procedure using cross-circulation (C. Walton Lillehei) |
1954 | First surgical correction of tetralogy of Fallot (C. Walton Lillehei) |
1955 | First successful use of the bubble oxygenator (Richard DeWall) |
1958 | First use of a small, portable battery-powered pacemaker (Earl Bakken) |
1963 | First human partial ileal bypass (Henry Buchwald) |
1966 | First clinical pancreas transplant (William D. Kelly and Richard C. Lillehei) |
1966–1968 | First prosthetic heart valves (Lillehei–Nakib toroidal disk in 1966, Lillehei–Kaster pivoting disk in 1967, Kalke–Lillehei rigid bileaflet prosthesis in 1968) |
1967 | Bretylium, a drug developed by Marvin Bacaner, saved the life of Dwight Eisenhower |
1967 | World’s first heart transplant (Dr. Christiaan Barnard, trained by C. Walton Lillehei) |
1968 | First successful bone marrow transplant (Robert A. Good) |
1969 | Invention of implantable drug pump (Henry Buchwald, Richard Varco, Frank Dorman, Perry L. Blackshear, Perry J. Blackshear) |
1976 | Medical Device Amendment to FDA Cosmetic Act |
1977 | First implant of St. Jude mechanical heart valve at University Hospital |
1978 | Human heart transplantation was performed at University |
1978 | Pediatric human heart transplantation was performed at University Hospital (Ernesto Molina) |
1988 | HDI/Pulse Wave® profiler founded (Hypertension Diagnostics, Inc., Jay Cohn, Stanley Finkelstein) |
1993 | Angel Wings® transcatheter closure device invented (Gladwin Das) |
1994 | First successful simultaneous pancreas–kidney transplant using a living donor (David Sutherland) |
1995 | Amplatzer® Occlusion Devices founded (AGA Medical Corp., Kurt Amplatz) |
1997 | First kidney–bowel transplantation (Rainer Gruessner) |
1999 | CardioPump Device evaluated (Keith Lurie, et al.) |
2000 | University’s Medical School has produced more family doctors than any other institution in the USA |
2003 | Robotic cardiac surgery performed at the University of Minnesota (Kenneth Liao) |
2005 | Launch of free-access website “Atlas of Human Cardiac Anatomy” (Visible Heart Laboratory; www.vhlab.umn.edu/atlas) |
2006 | Implant of left ventricular assist device using minimally invasive approach (Kenneth Liao) |

Fig. 25.6
Clarence Dennis with the first heart–lung machine at the University of Minnesota
Worldwide, one of the next major milestones in cardiac surgery was the first open-heart surgery performed using hypothermia, a procedure first attempted on September 2, 1952, by Drs. F. John Lewis and Richard Varco and colleagues at the University of Minnesota (Figs. 25.4 and 25.7). This procedure, proposed by Dr. W.G. Bigelow of Toronto, lowered the body temperature of patients 12–15 °F to reduce their blood flow, thereby reducing the body’s need for oxygen. Brain cells would die after 3–4 min at normal temperature without oxygen, but hypothermia allowed the University of Minnesota team (Drs. F. John Lewis, C. Walton Lillehei, Mansur Taufic, and Richard Varco) to successfully complete a 5½-min repair of the atrial septum of a 5-year-old patient. This was recognized as a significant landmark in the history of cardiac surgery; until this time, no surgeon had succeeded in opening the heart to perform intracardiac repair under direct vision. Hypothermia with inflow stasis proved to be excellent for some of the less complicated surgical repairs, but it was not a viable option for more extensive cardiac procedures. Major drawbacks of this approach at that time were the inability to rewarm a cold, nonbeating heart and the lack of clinical defibrillators [3].


Fig. 25.7
In this 1952 photo, Richard L. Varco (left) and F. John Lewis stand behind the hypothermia machine that they used during the world’s first successful open-heart surgery
From a historic perspective, another key milestone in cardiac surgery, though not accomplished at the University of Minnesota, occurred on May 6, 1953 when Dr. J. Gibbon closed an atrial septal defect using a pump oxygenator for an intracardiac operation. Although this first success with the pump oxygenator was well received, it aroused surprisingly little excitement or enthusiasm among cardiologists and cardiac surgeons at that time, likely because other centers had launched their own experiments with bubble oxygenators. Interestingly, Gibbon was never able to repeat his one clinical success; he ultimately became discouraged and did not use the pump oxygenator again.
During this era, “there was a common scenario, namely, good results with acceptable survival in the experimental animals but nearly universal failure when the same apparatus and techniques were applied to human beings [3].” Furthermore, it was written that “many of the most experienced investigators concluded with seemingly impeccable logic that the problems were not with the perfusion techniques or the heart lung machines [3]. Rather, they came to believe that the ‘sick human heart ’ ravaged by failure, could not possibly be expected to tolerate the magnitude of the operation required and then recover with good output, as occurred when the same machines and techniques were applied to healthy dogs [3].” It is important to consider that these experimental animals were typically healthy dogs and that anatomical differences between canine and human hearts may have been a significant distinguishing factor (see Chap. 6).
25.2 Cross-Circulation
Extracorporeal circulation by controlled cross-circulation was introduced clinically on March 26, 1954, after much animal experimentation (Figs. 25.4 and 25.8). The use of cross-circulation for intracardiac operations was an immense departure from established surgical practice at the time and was considered as a major breakthrough that motivated numerous innovations in the area of open-heart surgery [4]. The thought of taking a normal healthy human being into the operating room to provide donor circulation was considered “unacceptable and even immoral” by some critics. The risks to the donors included blood incompatibility, infection, air embolism (stroke), and/or blood volume imbalances.
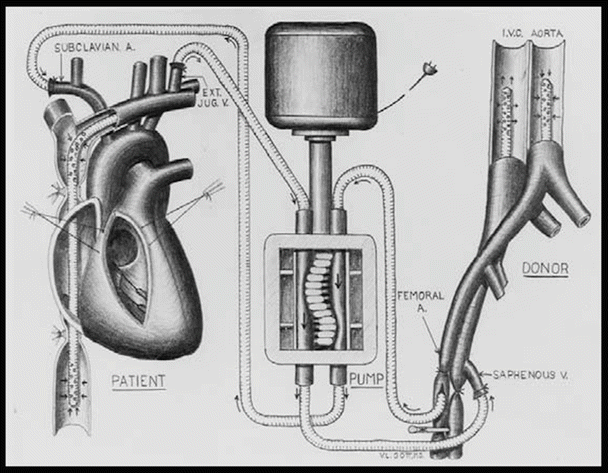

Fig. 25.8
Diagram of cross-circulation
From March 1955 onward, three additional bypass methods were introduced and successfully used, including (1) perfusion from a reservoir of arterialized blood, (2) heterologous (dog) lungs as an oxygenator, and (3) the Lillehei–DeWall disposable bubble oxygenator [3]. Yet, many believe that the single most important discovery that contributed to the success of clinical open-heart operations was the realization of the vast discrepancy between the total body flow rate thought necessary and what was actually necessary to maintain cerebral viability. Lillehei and his team are credited with applying the findings of two British surgeons (Andreasen and Watson) who identified the azygos factor —the ability of dogs to survive up to 40 min without brain damage when all blood flow was stopped except through the azygos vein. Specifically, Morley Cohen and Lillehei hypothesized that when blood flow was low, the blood vessels dilated to receive a larger share of the blood, while the tissues absorbed a much higher proportion of the oxygen as compared to normal circulation [3]. Previously, it was thought that basal or resting cardiac output at 100–160 ml/kg/min was safe maintenance during cardiopulmonary bypass. The azygos flow studies showed that 8–14 ml/kg/min maintained the physiological integrity of the vital centers, but Lillehei added a margin of safety and set his basic perfusion rate at 25–30 ml/kg/min. This approach reduced excessive complications of blood loss, excessive hemolysis, abnormal bleeding, and/or renal shutdown [3].
Altogether, 45 patients (aged 5 months to 10 years) underwent open-heart surgery with the cross-circulation approach at the University in 1954–1955; prior to these pioneering surgeries, such patients were considered to have lesions that were hopelessly unrepairable. Of this group, 22 (49 %) of the patients lived to be long-term survivors (greater than 30 years) and lead normal productive lives; 11 of the female long-term survivors subsequently gave birth to a total of 25 children who were free from any congenital heart defects. In addition, all 45 donors survived, with only one donor experiencing a major complication. At a more recent 53-year follow-up, 20 (44 %) of the original cross-circulation patients were living with no problems or significant limitations related to their original surgeries [5].
During this period of time, an intense competitive/collaborative relationship existed between the University of Minnesota and the Mayo Clinic (Rochester, MN, USA), the only other primary site where open-heart surgery was being performed. Lillehei recalled in his interview with G. Wayne Miller (author of King of Hearts) that the Mayo Clinic operated 7 days a week, so on Saturdays when Lillehei’s team was not scheduled for surgery, they would travel to the Mayo Clinic and watch Dr. John Kirklin and his colleagues (Miller, G.W., Transcriptions of audio tapes for King of Hearts, University of Minnesota Archives) [6]. Dr. Kirklin was successfully using a modification of the Gibbon heart–lung machine , and after observing his achievements, Lillehei began a slow transition away from cross-circulation and toward using a heart–lung machine of his own design (Figs. 25.4 and 25.9). In the beginning, Lillehei used the heart–lung machine for simpler, more straightforward cases and continued using cross-circulation for more complicated surgeries. Although its clinical use was short lived, cross-circulation is still considered today as one of the most important stepping stones in the development of the discipline of cardiac surgery.


Fig. 25.9
Mayo Clinic’s heart–lung machine was as big as a Wurlitzer organ; it cost thousands of dollars and required great skill to operate
25.3 Lillehei–DeWall Bubble Oxygenator
Importantly , John Gibbon, M.D., from Boston, invented the cardiopulmonary bypass procedure and performed the first intracardiac repair using extracorporeal perfusion in 1953. His bubble oxygenator, which looked surprisingly like a computer , was manufactured and financed by IBM. His reported achievements stimulated rapid development of the knowledge base and equipment necessary for both accurate diagnoses of cardiac disease and successful intracardiac operations. Yet at that time, it was recognized that the main problems with film oxygenators were (1) poor efficiency, (2) excessive hemolysis, (3) large priming volumes, and (4) development of bubbles and foam in the blood. All designs required blood flows of 2.2 l/m2/min, usually three to four units of blood for priming and another two units for the remainder of the circuit. Furthermore, after each use, the machine needed to be broken down, washed, rinsed in hemolytic solution, reassembled, resterilized, and reconfigured.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


