
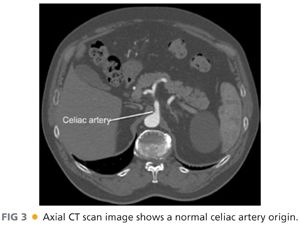
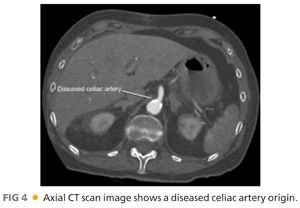
■ Computed tomography (CT) arteriography of the abdomen and pelvis, with arterial and venous pelvis, may provide additional useful information regarding the extent of aortic disease and other associated abdominal pathology (FIGS 3 and 4). Catheter-based arteriography alone may not identify significant arterial wall disease or the presence of aneurysmal lesions. However, the expense, contrast load, and radiation associated with complementary arteriographic imaging modalities may not be justified or appropriate in every patient, so anatomic information obtained from these examinations should be integrated into the operative plan on an iterative basis. Preoperative, imaging-based planning is combined with direct intraoperative assessment to create the most effective and durable revascularization possible for each patient.
■ Documentation of celiac, hepatic, splenic, and superior mesenteric artery patency is a mandatory prerequisite for these procedures. Significant stenosis of the celiac origin or hepatic or splenic artery occlusive disease will prevent successful renal revascularization from these arteries. Associated superior mesenteric artery disease also needs to be considered, particularly when the gastroduodenal artery provides significant collateral flow from the celiac plexus to the mesenteric bed. Renal artery anatomy, including branch vessel involvement and the presence of multiple renal arteries also needs to be documented.
■ Bilateral lower extremity vein mapping is also necessary to identify potential graft conduit. Standard vein mapping techniques, including imaging in a warm room with the patient in reverse Trendelenburg position, should be employed to ensure accuracy and reproducibility.
■ For selected patients, a more extensive preoperative evaluation for coronary artery or valvular disease should be considered. This may include both a transthoracic echocardiogram and cardiac stress evaluation. Selective pulmonary evaluation may be required in patients with chronic obstructive pulmonary disease (COPD)–associated respiratory compromise. Additional vascular assessments should be performed as indicated, including carotid duplex ultrasonography to assess the significance of carotid bruits identified on physical examination.
SURGICAL MANAGEMENT
Preoperative Planning
■ The indications for hepatic and splenic artery–based renal revascularization are similar to those for aorta-renal revascularization and are discussed elsewhere.1,4
■ Although aorta-renal bypass is most direct and generally most expeditious, extraanatomic renal revascularization may be preferable in selected circumstances as previously noted.
■ Review of preoperative imaging is performed to determine variant vascular anatomy, if present. Anatomy of the existing renal artery disease is assessed.
■ The hepatic–right renal bypass requires a conduit, preferably autogenous vein.
■ The spleno–left renal bypass may be performed with or without graft conduit. The native splenic artery is sufficient length, usually to extend directly to the left renal artery, when fully mobilized. When necessary due to variant anatomy, or prior inflammation or scarring around the pancreas, venous conduit can also be employed.
■ Planning for availability of duplex ultrasonography in the operating room (OR) will facilitate intraoperative confirmation of adequate target revascularization and renal perfusion.
Positioning
■ Patient is placed in supine position with both arms tucked.
■ A small bump is placed under the respective flank.
■ The operative field is prepped from the nipples to the knees.
TECHNIQUES
HEPATORENAL BYPASS
Placement of Incision
■ Optimal access is gained through a right subcostal incision extending from the midline to the tip of the 12th rib. In large or obese patients, the medial extent of the incision can be extended across the midline as a chevron (FIG 5).
■ When necessary, an upper midline incision may also provide sufficient exposure.
Hepatic Artery Exposure
■ The hepatoduodenal ligament is exposed by retracting the right lobe of the liver cephalad.
■ The right colon and duodenum are reflected anteriorly and to the left (Kocher maneuver). The small intestine is packed toward the pelvis with moist laparotomy pads.
■ The hepatoduodenal ligament is incised longitudinally. The hepatic artery is located in the porta hepatis medial to the common bile duct (FIG 6).

■ The gastroduodenal artery is identified as the first large branch coursing caudad and encircled with a silastic loop. The gastroduodenal artery should be preserved in the presence of superior mesenteric artery occlusive disease as it provides important collateral circulation to the small intestines.
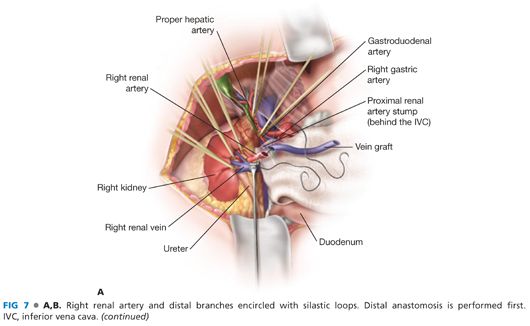
■ The hepatic artery is controlled proximally and distally with silastic loops (FIG 7).
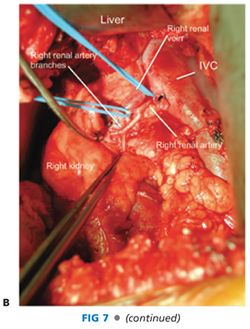
Right Renal Artery Exposure
■ The right colon and duodenum are reflected as detailed earlier to expose the inferior vena cava and right renal vein.
■ The right renal artery is located posterior and superior to the main renal vein. Depending on its position, the renal vein is retracted either cephalad or caudad. To ensure the main renal artery is exposed, the dissection should be carried to its aortic origin. This requires medial retraction of the inferior vena cava and division of lumbar veins when necessary.
■ The right renal artery is controlled using a silastic loop.
■ The main renal artery is exposed circumferentially and then distally to the three segmental renal artery branches. Each branch is identified and controlled with a silastic loop. This is a critical operative maneuver that excludes the presence of branch disease and ensures a successful renal artery revascularization (FIG 7).
Distal Anastomosis
■ The distal anastomosis is performed first to take advantage of the additional degrees of freedom provided by the mobile graft.
■ An appropriate length of greater saphenous vein is harvested from the thigh. The patient is heparinized 100 units/kg. The vein itself is reversed before placement.
■ The proximal renal artery is mobilized following its division from the aorta, at its origin. The proximal stump is oversewn with 5-0 polypropylene suture.
■ Redundant renal artery is trimmed distally from its origin until the disease-free segment is reached. The mobile renal artery is then transposed anterior to the inferior vena cava.
■ The vein graft and renal artery are spatulated and the end-to-end anastomosis created with continuous 6-0 polypropylene suture, knotted at opposite ends of the anastomosis to prevent purse-stringing. Alternatively, depending on renal artery diameter, eight interrupted sutures may be distributed circumferentially around the lumen. The smaller the renal artery diameter, the more advantageous the interrupted technique. Loupe magnification is necessary to ensure optimal results regardless of which suture technique is chosen (FIG 7).
■ Once the distal anastomosis is completed, the vein graft is oriented longitudinally to prevent twisting or kinking prior to completion of the proximal anastomosis.
Proximal Anastomosis
Hepatic artery
■ Small vascular clamps or removable clips are used to control the proximal and distal hepatic artery.
■ An arteriotomy is made on the hepatic artery and extended using Potts scissors.
■ The vein is spatulated and an end-to-side anastomosis is again performed with running polypropylene suture (FIG 8A).

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


