Chapter 54 Hemodialysis and Vascular Access
Temporary access to the venous system for the infusion of drugs or the transfusion of blood products has been a common practice for well over 300 years. Sir Christopher Wren, the great seventeenth-century English architect, is generally credited with developing an instrument for intravenous therapy in 1656, which was used for injecting drugs (opium and crocus metallorum) into the veins of dogs.1 It consisted of a cannula made from a goose quill with a pointed tip which permitted penetration of the skin and underlying vein. In 1663, Robert Boyle described and published Wren’s experiments and was the first person to extend intravenous infusions to humans, using prison inmates in London as subjects.2
The development of long-term cannulation of the circulatory system, however, was spurred by the introduction of a practical hemodialysis machine by Kolff in the mid 1950s.3 Initial enthusiasm for hemodialysis was blunted by the major technical problems associated with the need for repeated vascular access. Having to perform a cutdown on the artery and vein for each dialysis session, and then ligating these vessels at the termination of each procedure, essentially limited early hemodialysis to short-term therapy for acute renal failure. In 1960, the development of the Scribner arteriovenous shunt afforded relatively safe long-term access to the circulation,4 and repeated hemodialysis for the treatment of chronic renal failure became a reality.
As shown in Figure 54-1, the number of individuals requiring vascular access for hemodialysis continues to rise. By the end of 2008, there were nearly 550,000 people in the United States with end-stage renal disease (ESRD) from all causes, including more than 110,000 who received a diagnosis that year.5 In what appears to be an ongoing trend, the number of patients using hemodialysis increased approximately 3.5-fold between 1988 and 2008.5 Figure 54-2 demonstrates the number of vascular access device insertions from 1995 to 2010.6 Of note, the number of prosthetic access grafts inserted decreased while the number of arteriovenous fistulas increased. These changes are in line with the guidelines favoring fistulas published by the National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative and the National Vascular Access Improvement Initiative known as the Fistula First Breakthrough Initiative (FFBI).6 Specifically, the FFBI has supported an increase in the prevalence of national arteriovenous fistula rates from approximately 32% in 2003 to 55% in 2010.6 During this same period, there has been a decrease in chronic central venous catheter use rates.6 It should be noted, however, that the incident rate of central venous catheter (CVC) use in new hemodialysis patients remains to be high at over 80% in 2009.6
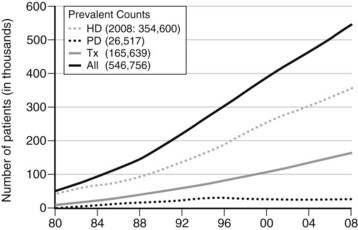
FIGURE 54-1 Number of treated end-stage renal disease patients in the United States by treatment modality, 1980 to 2008.
(From the U.S. Renal Data System, USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2010, Bethesda, Md.)
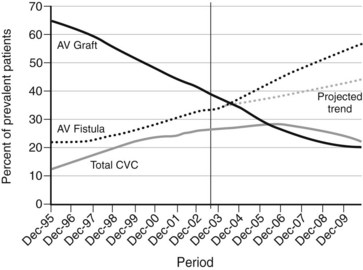
(From 2010 Fistula First Breakthrough Initiative Annual Report. Available at http://www.fistulafirst.org/AboutFistulaFirst/FFBIData.aspx. Accessed May 22, 2011.)
Short-Term Hemodialysis Access
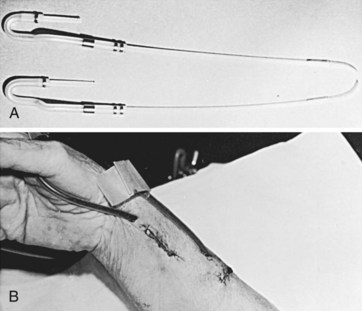
The external arteriovenous shunt described by Quinton and colleagues4 in 1960 consisted of a loop of silicone rubber tubing lying on the volar aspect of the forearm connecting two cannulas placed in the radial artery and a nearby wrist vein (Figure 54-3). Although quickly and widely adopted as a practical means of providing access in chronic renal failure patients, three major disadvantages to the long-term use of external shunts became apparent: (1) high risk of infection because of the likelihood of bacterial contamination at the tubing’s entrance sites in the skin; (2) frequent clotting owing to the small diameter of the silicone rubber and intravenous conduits; and (3) restriction of the patients’ daily activities, such as bathing, by the external appliance and the extra care necessary to prevent dislodgment or infection. Consequently, patency rates of external shunts were very low.8
Although acute hemodialysis was once conducted primarily with external shunts, these have now been replaced by percutaneously placed central venous catheters.9 This technique allows preservation of the vascular sites best suited for later construction of subcutaneous arteriovenous fistulas. The usual indications for hemodialysis by percutaneous venipuncture are (1) acute renal failure in which only a short course of dialysis is required; (2) immediately after surgery, while awaiting maturation of an internal fistula in patients with chronic renal failure; (3) patients with poorly functioning transplants who have thrombosed arteriovenous fistulas; (4) patients needing urgent transfer from peritoneal dialysis; and (5) treatment of poisoning.
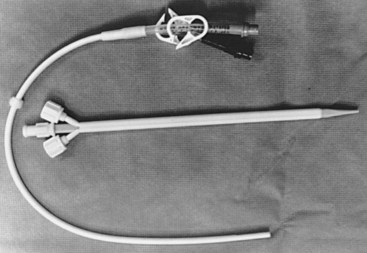
Short-term dialysis needs are met through the percutaneous introduction of a catheter into a central vein (Figure 54-4). The catheters are usually introduced via the Seldinger technique over a guidewire and can last up to several months.10 The catheter may be changed over a guidewire and the tip is cultured. Any drainage from the cutaneous entry site should also be routinely cultured. Thrombosis is prevented by continuous low-dose heparin infusion or an intermittent injection of heparin every 12 hours. Patients in stable condition can be given the option of going home with the catheter in place and receiving intermittent heparin injections for outpatient dialysis. Interestingly, patients with these indwelling catheters have a 40% incidence of moderate to severe ipsilateral subclavian vein stenosis on angiography.11 Placement of the catheter in the internal jugular vein is associated with less stenosis.
Central venous catheters are usually placed via the internal jugular and subclavian veins into the superior vena cava; this involves the usual risk of a percutaneous puncture of a major vein.12 The femoral vein may also be used if the previously mentioned veins are not patent and the catheter tip usually resides in either the common iliac vein or inferior vena cava; however, because of the risk of pelvic venous thrombosis, indwelling catheters in this location for a prolonged period should be avoided. Although the incidence of catheter sepsis is generally low, one series reported a 28% incidence of infection in catheters left in position more than 4 weeks.13 With adherence to standard antiseptic protocols, a catheter-related bloodstream infection rate below one episode per 1000 catheter days is possible, even without the use of antimicrobials.14
Long-term use of percutaneous vascular access is becoming a more common form of chronic hemodialysis in patients with no other site for hemodialysis. Using a silicone dual-lumen catheter with a Dacron cuff, a 65% 1-year catheter survival rate and an 18.5-month median length of catheter use have been reported.15 Although thrombotic complications occurred in 46% of patients, the use of thrombolytic therapy was successful in restoring catheter function more than 95% of the time. Catheter exit site infection in 21% of patients and bacteremia in 12% of patients were the other principal complications.
Autogenous Arteriovenous Fistula
The autogenous arteriovenous fistula, usually constructed by joining a superficial vein to an adjacent peripheral artery at the level of the wrist or in the mid forearm, remains the most dependable type of long-term vascular access. One long-term prospective study demonstrated a useful patency rate for first-time fistulas of 90% at 1 year and more than 75% at 4 years.16 In addition, revision of a failing fistula can extend its longevity. An autogenous arteriovenous fistula may be unsatisfactory, however, in patients (especially those with diabetes) with advanced atherosclerotic changes extending into the radial artery or in patients whose veins are too small, fragile, or thin walled for repeated needle punctures.
Radiocephalic Arteriovenous Fistula
The subcutaneous autogenous arteriovenous fistula was initially described by Brescia and coworkers in 1966.17 Readily accepted by nephrologists and surgeons, the Brescia-Cimino fistula, constructed of the patient’s own vessels, largely overcomes the disadvantages of infection and early clotting found with external arteriovenous fistulas. After formation of the fistula, arterial pressure is transmitted directly into the contiguous veins, resulting in dilatation and development of a hypertrophied muscular wall (Figure 54-5). This “arterialization” of the veins can take up to 6 weeks before vessels of sufficient size and wall thickness have developed to tolerate repeated venipuncture. During this postoperative period, hemodialysis may be accomplished using peripherally placed central venous catheters.
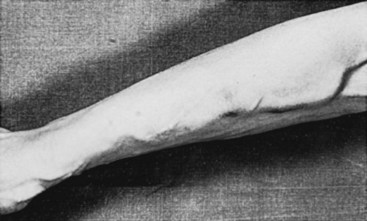
FIGURE 54-5 View of dilated forearm veins following construction of a Brescia-Cimino radiocephalic fistula.
Before the operation, the superficial arm veins, preferably in the nondominant arm, are distended and examined using a tourniquet applied to the upper arm to produce venous engorgement. All suitably sized veins are marked with an indelible pen. This is done so that if the fistula of choice fails immediately after construction, these markings can aid the surgeon in identifying other possible fistula sites. The ulnar and radial artery pulses are palpated, and if there is any uncertainty about their adequacy, the systolic pressure in each is measured with a Doppler probe. An Allen test, which predicts the ability of ulnar artery to support adequate circulation of the hand, should be performed to prevent symptomatic steal. The patient makes a fist and the examiner applies digital compression to the wrist, occluding both arteries; this is followed by pallor of the elevated and opened hand. Release of compression over the ulnar artery returns the hand’s appearance to normal if the blood supply is sufficient. In addition to visual inspection, many surgeons perform duplex examination of both arms to find the most suitable veins. In fact, the routine use of ultrasound vessel mapping has been recommended.18 In one study, ultrasound vessel mapping changed the preoperative plan in approximately 23% of patients.18
Local infiltration anesthesia using 0.5% to 1% lidocaine is usually satisfactory for construction of autogenous arteriovenous fistulas in the forearm or antecubital fossa. Although general anesthesia may be required in an extremely apprehensive or potentially uncooperative patient, a report on the effect of different types of anesthesia on blood flow during construction of a fistula showed that general anesthesia significantly decreases mean arterial blood pressure compared with local infiltrative anesthesia or regional block.19 In addition, brachial plexus block (supraclavicular approach) significantly increases brachial artery blood flow compared with local anesthesia. If available, an axillary block is an ideal anesthetic for vascular work in the forearm.
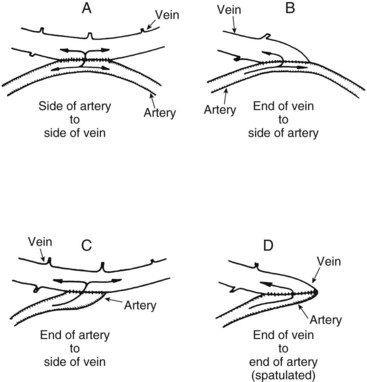
Four different anastomotic connections of artery and vein have been used (Figure 54-6), and each has its advantages and disadvantages:
1. Side-to-side anastomosis, with a fistula opening approximately 1 cm long, was the first procedure used. Technically, this is an easy anastomosis to construct and produces the highest fistula blood flow.20 It is also the most likely fistula to be associated with venous hypertension of the hand.21 This complication is moderated by the presence of venous valves that prevent reversal of venous blood flow in the hand, at least in the early months.
2. Vein end–to–arterial side anastomosis also decreases turbulence if constructed properly and results in the highest proximal venous flow with minimal distal venous hypertension.22 It is more technically difficult to construct than the side-to-side fistula, and fistula flow overall is somewhat decreased. If a branch vein is present, opening the inner aspect of the Y creates a generous oval patch to join to the side of the radial artery.
3. Arterial end–to–vein side anastomosis minimizes turbulence and distal steal of blood but may result in slightly lower fistula flows because it is subject to twisting of the artery during construction.
4. End-to-end anastomosis produces the least distal arterial steal and venous hypertension but has the lowest fistula flow of the four configurations.23
Proximal and distal control of the two vessels is gained by the application of small vascular clamps or a fine silicone rubber sling. The inability to pass a 3-mm or larger coronary dilator into the outflow vein has been associated with poor fistula maturation in our experience. The vessels are anastomosed in the desired configuration with 6-0 polypropylene sutures, with knots placed outside the lumen. One must ensure that when approximating the artery and vein, spiral rotation of these vessels does not occur. Before the anastomosis is finally closed, a check is made by gently passing a coronary artery dilator to detect any stenosis. This technique is especially useful to alleviate proximal arterial vasospasm in young patients. Hydrostatic dilatation with heparin-treated saline of a marginally small vein may aid in maintaining early patency.24 Any bleeding from the anastomotic site should first be controlled by simple pressure with a gauze swab for several minutes. Immediate suture repair can produce further bleeding sites and narrowing of the anastomosis.
Following discharge, the patient is instructed to keep the arm elevated for 24 hours and to avoid sleeping on the arm. Avoidance of constricting dressings, sphygmomanometer cuffs, and tight clothing is mandatory. Any swelling usually resolves over subsequent weeks. Generally, 4 to 6 weeks allows for adequate venous maturation for use. Puncturing the vessels before they are arterialized is often associated with hematoma formation, because the dilated veins are thin walled during the first few weeks.21 Although exercise of the forearm by squeezing a rubber ball to increase fistula flow and promote maturation of the arterialized veins has been advocated by some,24 others have reported that it has no benefit.25 Once the fistula is ready for cannulation, the buttonhole or constant-site technique should be considered in patients who self-cannulate.26
Brachiobasilic and Brachiocephalic Arteriovenous Fistulas
Brachiobasilic (with vein transposition) and brachiocephalic arteriovenous fistulas may be used to achieve upper extremity vascular access after failure of a more distal extremity arteriovenous fistula or if the cephalic vein at the distal forearm or wrist is inadequate. Patency rates of 80% at 3 years have been reported at these sites for chronic hemodialysis vascular access.27
Construction of the brachiocephalic fistula (Figure 54-7B) is preferred before brachiobasilic placement. The cephalic vein lies in a more superficial and accessible anatomic location on the anterolateral aspect of the arm compared with the basilic vein. The use of a transverse incision distal and parallel to the antecubital crease is recommended. Often a median antecubital vein, which drains into the cephalic vein, can be used and adds additional vein length for a tension-free anastomosis. Because more proximal vein diameters tend to be greater, brachiocephalic fistulas have a shorter time to maturity and longer primary patency when compared to radiocephalic fistulas.28 Attempts at more distal construction (Brescia-Cimino) should be performed whenever possible because upper arm access sites are limited.
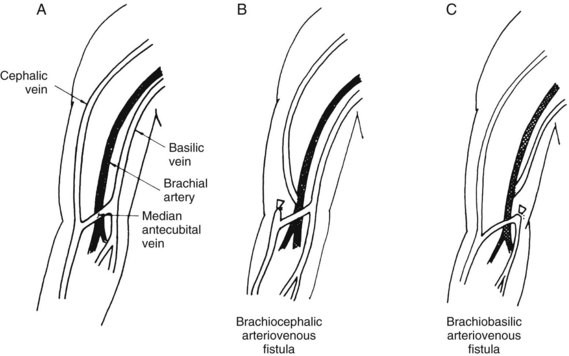
FIGURE 54-7 Normal anatomy (A) and brachiocephalic (B) and brachiobasilic (C) autogenous arteriovenous fistulas.
The brachiobasilic fistula (see Figure 54-7C) is constructed by identifying the basilic vein just anterior to the medial epicondyle of the humerus. Because of the deeper and more medial location of the basilic vein, this access site is usually avoided during phlebotomy and venipuncture. However, its location usually requires relocation in a subcutaneous tunnel running down the anterior aspect of the arm. If adequate basilic vein diameter (3 mm) is present distal to the elbow crease, the vascular anastomosis should be constructed in this location to maximize vein length for superficialization. This operation can be performed in one stage (vein mobilization, superficialization, and anastomosis) or two stages (anastomosis with delayed superficialization). Advantages of the two-stage approach include the ability to determine fistula maturation prior to vein mobilization, which requires a longitudinal incision in the upper arm, prolonged operative time, and increased postoperative pain. If a short basilic vein is present, length can be added for superficialization with either prosthetic polytetrafluoroethylene (PTFE) or autogenous (e.g., Artegraft, cryopreserved vein) conduits. Care must be taken during mobilization to avoid injuring the cutaneous nerves to the forearm, which lie adjacent to the vein. The proximal end of the vein remains in continuity with the axillary vein. The brachial artery is isolated in the antecubital fossa, and the end of the relocated vein is anastomosed to the anterior aspect of the artery in an end-to-side fashion at this level.
Vascular Grafts (Bridge Fistulas)
Successful long-term management of chronic renal failure frequently means that the patient outlives the usefulness of several serially constructed vascular access routes. When an autogenous arteriovenous fistula is no longer feasible, the use of a prosthetic conduit to form a bridge arteriovenous fistula is the best alternative. Arteriovenous grafts can be placed between almost any suitably sized superficial artery and vein. After implantation, these easily palpable conduits can be readily punctured by a needle; however, if possible, this should be delayed for about 2 weeks until the prosthesis has been incorporated into the patient’s subcutaneous tissue. Early puncture without careful hemostasis after needle removal may result in leaking of blood from the puncture site and formation of a perigraft hematoma.29
Sites for Arteriovenous Prosthetic Grafts
In the upper extremity, bridge arteriovenous grafts can be satisfactorily constructed between the radial artery and an antecubital fossa vein, between the brachial artery (in the antecubital fossa before its branching) and either the adjacent cephalic or basilic vein (U configuration loop), and between the brachial artery and the axillary vein (Figure 54-8). Construction of an upper-extremity bridge fistula is often more technically demanding than the creation of a femoral fistula, and its long-term patency is not as high.30,31 This is generally attributed to the larger vessels and greater blood flow in the thigh. The risk of infection and distal limb ischemia is less in fistulas constructed in the upper extremity,29 however, and this is the preferred site. Patients with claudication or an ankle arterial pressure less than 80% of that at the wrist might not be suitable for thigh fistulas, because the proximal steal of blood through the fistula is likely to increase ischemia in the leg.32 Therefore upper extremity fistulas are particularly well suited for elderly patients with significant atherosclerosis in the lower extremities. Obese patients in whom perspiration or dermatitis involving the groin skinfolds may increase the likelihood of infection should have an arm fistula. Incontinence is a relative contraindication for implantation of the graft in the upper thigh.
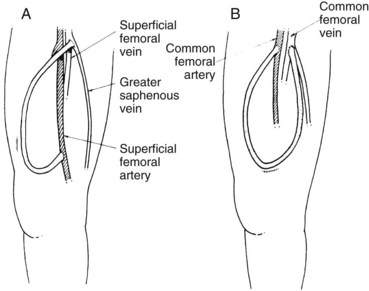
The arterial anastomosis in the lower extremity should be to the superficial femoral artery, immediately proximal to either the adductor canal or its more cephalad portion (Figure 54-9A). If the superficial femoral artery is occluded, the common femoral artery may be used (see Figure 54-9B), with the understanding that if it becomes infected and ligation is subsequently necessary, leg ischemia may ensue. At times, patency of a short segment, including the origin of the superficial femoral artery, can be reestablished and used for the arterial anastomosis. The venous anastomosis is made to the proximal saphenous, common, or superficial femoral vein.
Historically, the site selected for the initial placement of an arteriovenous graft was in the forearm, from the distal radial artery to the cephalic or basilic vein in the antecubital fossa.33 The graft was anastomosed in an end-to-side fashion to the distal radial artery, tunneled along the lateral aspect of the forearm, and then anastomosed end to side to the largest vein in the antecubital fossa. In positioning the graft, one had to ensure that the patient’s arm would rest comfortably when receiving hemodialysis.
Currently, more common vascular access sites in the upper extremity include brachiocephalic or brachiobasilic loop fistulas in the forearm and brachioaxillary fistulas in the upper arm. Loop fistulas placed in the forearm allow a large area of graft to be available for needle puncture, whereas the brachioaxillary fistula, which curves over the lateral aspect of the upper arm, has several sites for venous anastomosis on the axillosubclavian segment. Upper extremity loop grafts have also been found to have significantly higher patency rates at all time intervals than do straight upper extremity grafts.34 Upper-extremity procedures can be performed using an axillary nerve block or local infiltration anesthesia with sedation.
Arteriovenous grafts in the thigh are usually constructed with the patient under a spinal or general anesthetic, although in cooperative patients, a local infiltration technique can be used. Placing the arterial origin of the conduit just proximal to the adductor canal portion of the superficial femoral artery is often advisable so that if a vascular complication should cause occlusion of the artery, adequate collateral channels will provide filling of the popliteal segment. The end-to-side arterial anastomosis should be oblique, and the graft should leave the artery at an angle to minimize turbulence. The venous anastomosis is also performed in an end-to-side fashion and as obliquely as possible. This method is used to counteract any purse-string effect of the suture, as well as buildup of fibrin and fibrous tissue at the venous anastomosis, which commonly causes late graft thrombosis. A vascular steal phenomenon, with reversal of flow in the distal superficial femoral artery, is common in bridge fistulas in the lower extremity and can lead to symptoms of limb ischemia.35 Fortunately, most patients with steal do not have symptoms, because dialysis patients are often fairly inactive.
The femorosaphenous bridge fistula is curved subcutaneously over the lateral aspect of the thigh and anastomosed to the proximal saphenous vein. The caudal portion of the saphenous vein may be ligated to prevent retrograde venous flow, although venous hypertension in the lower extremity is not a problem with a patent iliofemoral system. Another lower-extremity access configuration is the loop fistula placed in the groin from the common femoral or very proximal superficial femoral artery to the femoral vein. The high blood flow rate (>1000 mL/min) can lead to a significant increase in cardiac output. The possibility of limb loss in the event of infectious complications and the increased risk of infection make this site less desirable.35
With the longer survival of chronic hemodialysis patients, the surgeon may be asked to evaluate a patient who requires vascular access but whose extremity access sites have all been expended. In this circumstance, a more central location, such as a bridge arteriovenous fistula placed between the axillary artery on one side and the axillary vein on the other side, has been used successfully.36 The grafts are of fairly large diameter, so they are easy to cannulate; flow is reported to be excellent, and despite the location of the access site on the anterior chest wall, patients adapt promptly.37 The major drawback of central access sites is that when complications occur, they are serious and more difficult to manage.
Materials for Prosthetic Arteriovenous Bridge Grafts
Both biological and prosthetic materials have been used in the creation of arteriovenous bridge fistulas for hemodialysis since this modality was introduced in 1969.38 Although saphenous vein, bovine heterografts, human umbilical vein, cryopreserved homografts, and Dacron velour grafts have all been tried during the last 4 decades, only expanded PTFE grafts have had an extended period of observation. Although one report on the use of autologous tissue-engineered vascular grafts in high-risk patients demonstrated primary patency in 78% of patients 1 month after implantation and 60% of patients 6 months after implantation, larger trials are needed to better determine the safety and efficacy of these grafts.39
Since its initial introduction as an alternative material for the creation of arteriovenous bridge fistulas in 1976,40 expanded PTFE has become the most commonly used material. Much of its popularity stems from the fact that it is easy to handle, requires no preclotting, is widely available, has a long shelf life, and has relatively high patency rates with secondary revisions. PTFE bridge fistulas are consistently reported to have 12-month secondary patency rates of more than 70%.41 In a large comparative clinical study comprising 187 graft placements, 36-month patency rates of PTFE grafts were significantly greater than those of bovine heterografts (62% vs. 24%).42 Forty-eight–month patency rates of 43% to 60% have been reported.43,44 However, multiple procedures for revision are usually required to maintain patency, with one study reporting an average of one operation for revision required every 1.1 years (range, 1 to 16 revisions per graft).45
Thrombosis of the conduit is a relatively common event in PTFE bridge fistulas, with figures ranging from 7% to 55%.43,44 Endovascular techniques such as thrombolysis, percutaneous mechanical thrombectomy, and angioplasty allow for reestablishment of graft flow and function. Surgical revision with Fogarty thrombectomy and patch angioplasty of the stenotic outflow vein is also effective.44 Infection of PTFE is not uncommon, with one report of 80 AV grafts monitored for 30 months showing an overall incidence of infection of 19%, with 67% of these infections occurring during the initial 4 months of use.46 Of the infected grafts, 73% required excision, and the remainder were treated successfully with antibiotics. The most common type of graft infection today occurs at needle puncture sites. Pseudoaneurysm at needle puncture sites develops in approximately 5% of fistulas.43
Recently, heparin-bonded ePTFE grafts have been introduced in reports of increased patency compared with conventional ePTFE grafts.47 The long-term advantages and complications of heparin-bonded ePTFE grafts are not yet known.
Pediatric Vascular Access
Dialysis (either hemodialysis or peritoneal dialysis) with subsequent renal transplantation is the preferred therapeutic regimen for ESRD in children. Transplantation is usually attempted as quickly as possible, because in general, children tolerate dialysis poorly; they frequently develop severe growth retardation (not reversible by transplantation), failure of maturation, renal osteodystrophy, and psychosocial problems.48,49 In addition, long-term hemodialysis is, at best, difficult in children younger than 10 years and is extremely difficult in those younger than 5 years.
Short-term hemodialysis in children can be performed in a variety of ways with good results. Newborn or premature infants can be dialyzed via direct cannulation of the umbilical vessels using a 5- or 8-French catheter. Single-cannula hemodialysis can be used in older children in whom the superior or inferior vena cava has been cannulated with either the Seldinger technique or direct venous cutdown.49
Long-term vascular access for hemodialysis in children weighing less than 20 kg can be accomplished by placing a central venous catheter, inserted as described previously.50 When external access is used, the recommended blood flow rate should be at least 3 to 5 mL/kg/min in most patients.51 In addition, creation of an autogenous arteriovenous fistula between the brachial artery at the elbow and an antecubital vein using microsurgical techniques has been done in children weighing between 10 and 23 kg, with excellent results.52,53
For long-term hemodialysis access in children weighing more than 30 kg, an internal form of access, either autogenous fistula or bridge fistula, should be attempted. For children weighing 20 to 30 kg, the type of access attempted must be individualized according to the size of their vessels. Long-term access in children weighing greater than 20 kg should be seriously considered when transplant wait list times are anticipated to be greater than 1 year.51 A Brescia-Cimino autogenous arteriovenous fistula can be created in children weighing more than 30 kg without much difficulty, and with patency rates of approximately 80% at 12 months. The use of microsurgical techniques enhances the patency rate, especially in children with small vessels.53 Bridge fistulas of PTFE have also been used in children, with acceptable patency rates.
The usual types of complications and their rates of occurrence with both autogenous and bridge fistulas in children approximate those in adults. However, one of the most common complications of hemodialysis in small children is convulsions, occurring in up to 30% of patients.54 This is probably the result of two factors: the use of overly efficient dialysis and the greater sensitivity of children to changes in osmolality. Convulsions can probably be avoided by tightly regulating the efficiency of the dialysis procedure based on the child’s body weight. One major disadvantage of the use of internal vascular access fistulas in children is the physical and psychological pain of repeated needle punctures, which may require much time and counseling to overcome.
Vascular Access Complications
Infection
Infection causes 15% to 36% of all deaths in dialysis patients, exceeded only by cardiovascular disease.55 It also accounts for 20% of inpatient hospital admissions in these patients.55 Many of the systemic infections encountered in these patients are direct complications of infection at the site of hemodialysis access. Two large dialysis centers found an incidence of 0.11 septic episodes per patient-dialysis-year related specifically to the vascular access site.56 This represented more than 73% of the total number of septic episodes encountered.
The elevated rate of sepsis associated with hemodialysis vascular access sites is partially due to the deficient immune defense mechanisms in patients with chronic renal failure and the consequent increase in infection risk.57 The bacterial phagocytic and killing ability of polymorphonuclear leukocytes also decreases by nearly 50% in patients with chronic renal failure.58 Lymphocytes in chronic renal failure exhibit suppressed cellular immunity, and inhibition of lymphocyte transformation, which is unaffected by dialysis, has been detected.59 In addition, the serum of uremic animals contains a nondialyzable inhibitor of the mixed lymphocyte reaction, which is probably a glycoprotein and distinct from either α-macroglobulin or immune complexes.60 The actual ability of the animal to produce antibodies when antigen stimulated, however, does not appear to be depressed in chronic renal failure.61
In addition to alterations in host defense mechanisms, other factors contribute to the increased propensity of patients to develop infection when requiring long-term hemodialysis. Poor healing of surgical wounds is a recognized consequence of renal failure and may result in wound infections. Measurement of the bacterial colonization rate of patients receiving hemodialysis revealed that 62% of these patients carried Staphylococcus aureus in their oropharynx or nasopharynx or on the skin, and 65% of those patients with positive cultures developed infections in their hemodialysis access sites.62 Furthermore, 30% of the dialysis staff carried S. aureus, whereas only 11% of normal controls had positive cultures. In the same study, more than 70% of all infections encountered were caused by S. aureus. In a separate study, it was found that the 12-week mortality for patients with S. aureus bloodstream infections was 20.2% and for those with nonbloodstream infections was 15.7%.63 Strict antisepsis is the best means to prevent S. aureus colonization and reduce the risk of vascular access site infection.64 In a prospective evaluation of patients undergoing hemodialysis at multiple outpatient facilities, it was noted that patients with AV grafts, tunneled catheters, and temporary catheters had a significantly higher risk of developing infection than those with native AV fistulas.65 Specifically, patients with AV grafts had a 2.2-fold higher risk, patients with tunneled catheters had a 13.6-fold higher risk, and patients with temporary catheters had a 32.6-fold higher risk.65
Although infection of an autogenous arteriovenous fistula is unusual, it can occur. Repeated puncture of the fistula can result in formation of a hematoma that can subsequently become infected by skin microflora. The method of cannulation can also affect the incidence of infectious complications in autogenous fistulas.66 A quality improvement study comparing infectious complications following conversion to buttonhole cannulation concluded that with stringent adherences to a protocol for the buttonhole technique, as well as appropriate education and training, this technique is associated with a decrease in infectious morbidity.66 In addition, the anastomotic site of the fistula itself can become infected, resulting in an endovasculitis with subsequent septicemia and metastatic abscess formation. Treatment generally consists of therapeutic courses of appropriate antibiotic agents coupled with local measures such as drainage of a perifistular abscess from an infected hematoma. Rarely, the fistula anastomosis may have to be dismantled, and the vessels are ligated in the presence of an infection-induced anastomotic pseudoaneurysm.
AV grafts placed for vascular access are susceptible to multiple sources of infection. Contamination from skin flora can occur during implantation and is more frequent when the fistula is placed in the thigh than when it is located in the upper extremity. In part, this is caused by the greater difficulty in preparing a sterile surgical field on the medial thigh and inguinal skinfold.67,68 Direct inoculation of the graft by needle puncture through inadequately prepared skin also occurs, as well as inoculation of hematomas, resulting in perigraft abscess formation.
The type of material in AV grafts also affects the infection rate, with autogenous saphenous vein fistulas demonstrating few, if any, infections and biological conduits (human umbilical vein graft, bovine heterograft) being particularly susceptible to aggressive infections; synthetic conduits are also susceptible to infection by low-virulence organisms.69 The newly implanted prosthesis is particularly susceptible to infection; however, tissue incorporation and neointima formation confer increased resistance to infection.70 A 2-week delay in initiating hemodialysis using AV grafts allows tissue incorporation of the prosthesis and development of a neointima. Disruption of an infected graft anastomosis (Figure 54-10) can occur at any time during the course of a prosthetic infection and does not appear to be influenced by incorporation.69
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree