Hemodialysis Access Maintenance and Salvage
Dirk M. Hentschel
Dialysis accesses are dynamic connections between an artery and a vein, either directly (= autogenous) or by use of a prosthetic or biologic (bio-) grafts (nonautogenous). To support dialysis, an access has to provide flows slightly higher than the typical blood pump speed (250 to 500 mL/min), and allow for repeated needle insertion as well as safe needle removal.
Once an access can be reliably used for dialysis it is considered to be “mature.” Interventions performed between creation or placement of the access until then are typically performed for “failure of maturation.” A majority of accesses in the United States require interventions prior to usability.
With their dialysis access, chronic kidney disease (CKD) patients also acquire “chronic access disease” as most stenoses are recurrent, outflow stenoses usually with higher frequency than inflow stenoses. Typical locations for access stenoses are the juxta-anastomosis for forearm radial-cephalic autogenous accesses, the cephalic arch for upper arm brachial-cephalic autogenous accesses, the swing point for upper arm transposed brachial-basilic or brachial–brachial accesses, and the venous anastomosis and immediate outflow vein for grafts. Central vein stenoses are often associated with previous insertion central venous catheters, as well as leads from cardiac rhythm devices. Outflow and central vein stenoses can lead to high intra-access pressures associated with access enlargement and bleeding, and if left untreated may cause thrombosis of the access. Angioplasty and stent or stent graft placement are endovascular options to treat access stenoses. Thrombectomies can reestablish access flow in most instances; large thrombus burdens may require the use of adjunct techniques such as mini-incisions or “sheath-hole” access to extricate thrombus with instruments under direct manual palpation.
Enlargement of needle insertion sites with loss of skin integrity is often a limiting factor for access longevity. Drivers of this process are (a) focal needle insertions sites (“one-site-itis”), (b) elevated intra-access pressures due to recurrent outflow stenoses, very high blood inflow, or both, and (c) patient-specific conditions (e.g., collagen vascular disease). Banding and imbrication of the inflow are techniques to reduce flow and intra-access pressures, and retard access enlargement. Enlarged accesses can be reduced in size by aneurysmorrhaphy with excision of deteriorated overlying skin.
Long, multistenotic segments in the upper arm cephalic vein are replaced with interposition grafts while at the same time treating outflow stenosis (stent/stent graft) and regulating inflow with banding as needed.
Long, multistenotic segments in the upper arm cephalic vein are replaced with interposition grafts while at the same time treating outflow stenosis (stent/stent graft) and regulating inflow with banding as needed.
Table 20.1 Physical Examination at 4 to 6 Weeks | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Access interventions are guided by the combination of (1) clinical signs and symptoms, (2) physical examination (Tables 20.1 to 20.3), and (3) diagnostic imaging.
Common clinical complains from the dialysis unit are:
“needles are pulling clots”—This may indeed mean that there is ongoing thrombosis in a needle insertion segment, but this is rare. More commonly, when the tip or the back-hole of the dialysis needle is not properly within the lumen of the access vessel, there will be slow leaking of blood into adjacent tissue at this site with appropriate thrombus formation that may then extend into the needle and can be aspirated. This is a sign of difficulty with needle insertion. Most commonly, augmentation of the access vessel despite occlusion of the outflow is impaired, either due to a collateral or because inflow is low in the context of an anastomotic or juxta-anastomotic stenosis.
“vessel collapses,” “access spasms”—During the first needle insertions into an access or a new segment of an access there may be venospasm at the needle insertion site. Typically this resolves over the course of 20 to 30 minutes. However, intermittent collapse of the inflow vessel during the entire dialysis treatment may be a sign of access flows near or below dialysis pump speed.
“long bleeding,” “bleeding on a nondialysis day”—Once true defects in the skin coverage of an access and excessive anticoagulation have been excluded, one should suspect elevated intra-access pressures and look for other signs of an outflow stenosis or very high inflow.
Table 20.2 Physical Examination of Mega-Access | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
The physical examination is instrumental in guiding imaging evaluation as well as correlating observed angiographic abnormalities with clinical dysfunction to counter the “oculo-manual” reflex for intervention (Fig. 20.1, Tables 20.1 to 20.3). Each dialysis access presents with unique features as a result of smallest inflow diameter (= flow
limiting/regulating segment), blood pressure, outflow capacity, and presence of collaterals. To capture all (patho-)physiologic components of a given access by physical examination we suggest to evaluate the following five categories: pulsatility, flow murmur/bruit, thrill, augmentation, and collapse of access against gravity. Repeated examinations and correlation of physical findings with angiographic features as well as flow measurements will lead to useful mastery of this skill.
limiting/regulating segment), blood pressure, outflow capacity, and presence of collaterals. To capture all (patho-)physiologic components of a given access by physical examination we suggest to evaluate the following five categories: pulsatility, flow murmur/bruit, thrill, augmentation, and collapse of access against gravity. Repeated examinations and correlation of physical findings with angiographic features as well as flow measurements will lead to useful mastery of this skill.
Table 20.3 Physical Examination of Dysfunctional Accesses—Most Common Site of Stenosis | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Pulsatility describes the force of access expansion during systole and the degree of softening during diastole. Very high blood pressures will suggest increased pulsatility, but the access softens remarkably during diastole. An outflow stenosis will lead to increased pulsatility and reduced softening during diastole. An inflow stenosis will blunt the systolic component and create the impression of an “empty” access during diastole, unless there is a coexisting outflow stenosis. Pressure measurements in the body of accesses (needle insertion segment) with “normal” pulsatility typically are in the 20 to 25 mm Hg range for forearm accesses, and 25 to 30 mm Hg for upper arm accesses. Suggested categories: none, decreased, normal, increased.
The audible flow murmur can be characterized by pitch and continuity. A change in pitch toward higher frequency is typical at the site of a stenosis due to accelerated flow velocity at this site. A discontinuous flow murmur indicates that during diastole flow is so low that no audible shear force is created. This is the sign of a severe inflow or outflow stenosis. Typically, the stenotic inflow murmur is faint (like a whistle), while the stenotic outflow murmur can be coarse and loud (akin to a wood saw).
A thrill is palpable through the skin when the vessel is close enough to the surface and the flow high enough in relation to the diameter of the vessel to create vibration of the vessel wall. A thrill can be the sign of a well-developed access, usual in the inflow segment, dissipating as the access vessel increased in diameter, branches and takes a deeper course. It is usually continuous with slight pronunciation during systole. However, a discontinuous thrill can be found with severe stenosis. An isolated thrill is also found focally immediately after a stenosis. The differentiation from a “healthy” thrill can be made by documenting a change in pulsatility at the site of the focal thrill, increased retrograde (inflow side) and decreased antegrade (outflow).
Augmentation is the firming up and enlargement of the body of the access (where needles are inserted) with occlusion of the outflow. Without proper augmentation needle insertion will be complicated by perforating injuries and hematomas. An inflow stenosis will impair augmentation as will side-branches and collaterals between the occluding finger/tourniquet and the inflow. The location of side-branches can be elucidated by moving the occluding finger closer toward the anastomosis until augmentation is achieved. With several collaterals this may be a staged phenomenon. Suggested categories: absent, weak, moderate, and strong.
Collapse of the access with arm elevation (against gravity) is a measure of inflow and outflow capacity match or mismatch. A forearm access typically displays complete collapse while upper arm accesses typically show only partial collapse. Outflow stenoses or very high inflow will decrease the degree of collapse, banding of an upper arm access or a natural flow limiting stenosis may lead to complete collapse of an upper arm access.
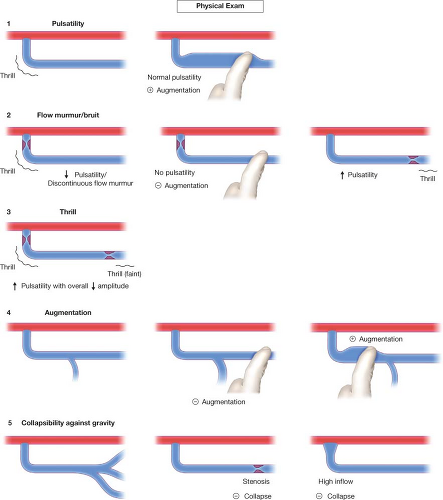
Figure 20.1 The physical examination of dialysis accesses includes five components: pulsatility, flow murmur/bruit, thrill, augmentation, and collapsibility against gravity.
Enlarged needle insertion sites (and any sites of suspected skin thinning) are best examined while occluding the inflow: the completely empty access allows palpation of firm, layered thrombus inside aneurysms as well as better appreciation of the thickness of the overlying skin by rolling it between thumb and index finger.
Diagnostic imaging includes use of ultrasound (US) as well as angiography using iodinated contrast agents or CO2 gas. US can accurately determine the depth of an access, demonstrate intraluminal thrombus and provide estimates of access flow by calculating flow in the inflow artery. Angiography provides patterns of flow distribution, unmasking relevant side-branches and stenoses. During angiography access flows can be directly measured using special endovascular catheters based on a modified Fick’s equation.
Guided by physical examination, angiographic studies and interventions can be performed with minimal contrast volume (5 to 10 mL) which typically do not jeopardize renal function even in patients with advanced CKD. We dilute contrast 1:1 with normal saline for central venous imaging and 1:3 with normal saline for peripheral images, and perform pullback angiograms from central to peripheral (the usual access entry site). Diluted contrast allows for better appreciation of vascular details otherwise hidden by dense contrast.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


