Heart Valve Repair and Replacement
Valvular heart disease can be treated in a variety of ways:
valve replacement, in which an artificial (prosthetic) heart valve is implanted surgically to replace an abnormal (regurgitant or stenotic) valve
valve repair, in which a regurgitant valve is corrected surgically, preserving the original valve rather than replacing it
percutaneous techniques, which include percutaneous balloon valvuloplasty (in which a stenotic valve is ‘stretched’ with a balloon) and, more recently, transcatheter aortic valve implantation (in which an abnormal aortic valve is replaced with a new valve deployed percutaneously rather than with cardiothoracic surgery).
It is important to be aware of the different valvular procedures that patients undergo, as patients will require a follow-up echo study to confirm the success of the procedure and in some cases to monitor their valve for any subsequent dysfunction. Following valve surgery, a baseline echo is usually undertaken 3-12 weeks after the operation. This allows time for any post-operative anaemia to resolve (which would otherwise cause a hyperdynamic high-output state, affecting quantitative measurements) and for left ventricular function to improve, as well as making the scan more comfortable for the patient once their chest wound has healed.
Following the baseline echo, for mechanical valves further ‘routine’ follow-up scans are not usually necessary unless there is clinical cause for concern. For biological valves, routine scanning is not usually needed in the first 5 years after the initial baseline echo, but beyond 5 years routine annual follow-up scans are recommended to monitor for any dysfunction.
PROSTHETIC VALVES
Each year approximately 6000 people in the UK (and 60 000 in the USA) undergo surgery to implant a prosthetic heart valve. Prosthetic valves fall into one of two categories:
Mechanical valves – in which the valve is constructed using artificial materials.
Biological valves – in which the valve contains biological material, derived either from a natural valve or fashioned from pericardium. Biological valves are also sometimes called ‘tissue valves’ or ‘bioprosthetic valves’.
Before undertaking an echo assessment of a prosthetic valve, it is essential to know the type of valve implanted. The request form must therefore include:
the type of prosthetic valve (e.g. biological, mechanical) and its specific name (e.g. Bjork-Shiley)
the size of prosthetic valve (which is its internal diameter, stated in mm)
the date of implantation
current clinical details (e.g. new diastolic murmur)
a specific question to be addressed (e.g. valve dehiscence?).
Details of the type of valve can usually be obtained from the original operation note.
Echo assessment of mechanical valves
A mechanical valve consists of three parts: the sewing ring (which is like the ‘annulus’ of the valve, used by the surgeon to sew the valve into position), the occluder (the moving part of the valve which opens and closes during the cardiac cycle) and the retaining mechanism (which is attached to the sewing ring and holds the occluder in position).
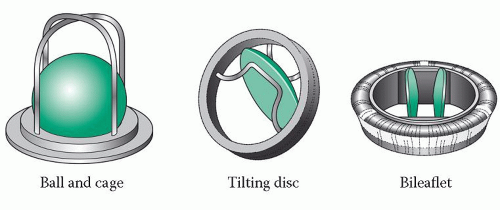
There are three types of mechanical valve (Fig. 22.1):
ball and cage valves, consisting of a silastic ball occluder which can move up and down within the cage-like retaining mechanism – this was the earliest type of mechanical valve, introduced during the 1960s
tilting disc valves, in which a single disc occluder tilts within its occluder
bileaflet valves, in which two semicircular disc occluders open and close on hinges – these are now the most commonly used prosthetic valves.
The advantage of mechanical valves is their long-term durability (although some earlier valves were prone to catastrophic failure). The main disadvantage of mechanical valves is that, because they are constructed from artificial materials, they can be a source of thrombus formation. Patients with mechanical valves therefore require lifelong anticoagulation with drugs such as warfarin, which can be a major drawback, particularly in patients at risk of bleeding or women of child-bearing age who wish to become pregnant.
Mechanical valve structure
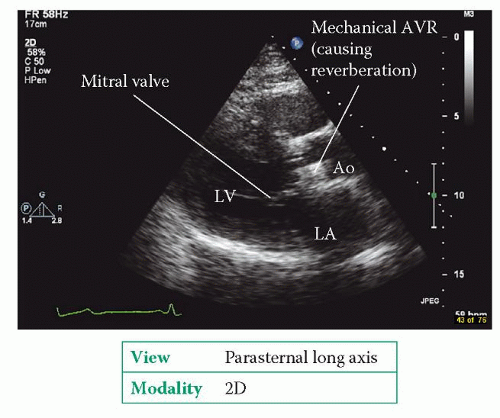
Mechanical valves can be challenging to assess on echo because of the reverberation caused by the materials in the valve (Fig. 22.2). Mitral prostheses are usually best assessed from the apical window, aortic prostheses from the apical and parasternal windows. Transoesophageal echo (TOE) can help, particularly for prosthetic valves in the mitral position (see box).
As far as possible, examine the structure of the mechanical valve, asking the following questions:
Is the valve well seated, or does it appear to be ‘rocking’? A rocking valve prosthesis indicates a degree of separation (‘dehiscence’) of the valve’s sewing ring from the rest of the heart – look carefully for associated paravalvular regurgitation.
Is there a normal range of movement of the valve occluder(s)? Occluder motion can be obstructed by thrombus or pannus (excessive fibrous or ‘scar’ tissue around the valve). Obstruction to occluder opening causes stenosis, while obstruction to occluder closure causes regurgitation.
Are there any masses associated with the valve, and are the masses mobile or immobile? Pannus is an immobile mass, whereas thrombus or vegetations are usually (but not always) mobile. Pannus typically occurs 5 years or more postsurgery, although it has been found as early as 6 months post surgery. Thrombus or vegetations can occur at any time. Prosthetic valve masses usually require a TOE study for full characterization.
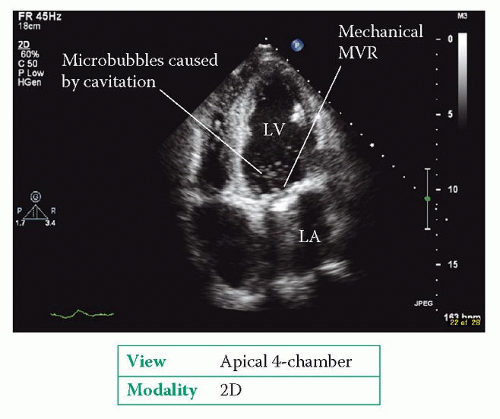
Sometimes very small bubbles are seen near a mechanical valve just as its occluder closes (Fig. 22.3). These microbubbles are caused by cavitation of blood by the occluder, and are regarded as a harmless finding.
If the valve prosthesis cannot be imaged adequately, state this in your report so that appropriate alternative imaging can be arranged.
TOE AND MECHANICAL VALVE ASSESSMENT
TOE can play a valuable role in the assessment of mechanical valves, particularly in the mitral position. TOE provides good resolution of the left atrium and the mitral valve, and so can provide useful information on the function of a mechanical mitral valve prosthesis. TOE is less useful for imaging mechanical aortic valves, particularly when a mechanical mitral valve is also present.
Mechanical valve function
Forward flow
As for native valves, assess forward flow by measuring:
gradient (peak and mean) – but beware the problem of pressure recovery (see box)
pressure half-time (for mitral prostheses)
effective orifice area (EOA).
For prosthetic aortic valves, EOA is measured using the continuity equation (p. 159). Be particularly careful when measuring the left ventricular outflow tract (LVOT) diameter – it is important to be sure that you’re measuring the true LVOT diameter, and not the diameter of the prosthetic valve itself. When measuring the velocity time integral of flow in the LVOT using PW Doppler, it is important to place the PW sample volume 0.5-1.0 cm proximal to the valve (if it’s too close to the valve, it may lie within the region of subvalvular flow acceleration).
For prosthetic mitral valves, EOA is also measured using the continuity equation (p. 176), as long as no more than mild mitral or aortic regurgitation is present. Do not use the pressure half-time method to estimate prosthetic mitral valve EOA (although the pressure half-time itself can be used to compare forward flow between follow-up visits).
Bear in mind that the flow through a mechanical valve tends to be very different to that through a native valve. For instance, forward flow through a bileaflet disc valve will consist of three individual jets. Careful Doppler interrogation is necessary to ensure that you identify the peak velocity.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree