Chapter 70 Hand Surgery
Basic Anatomy
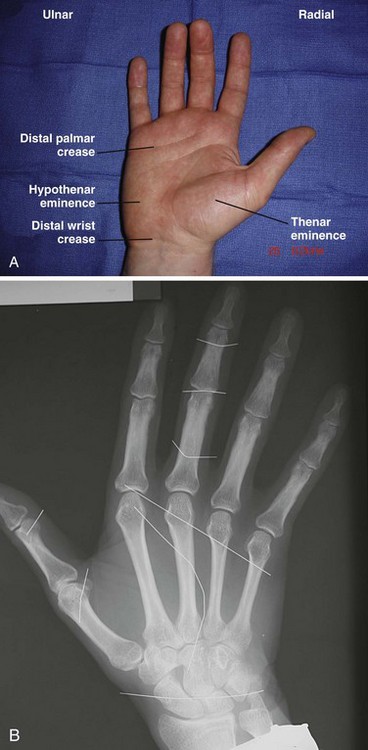
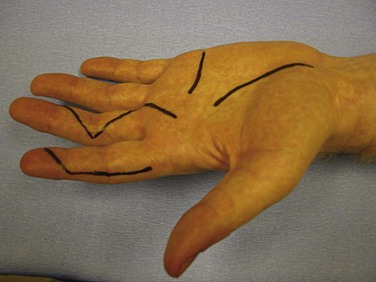
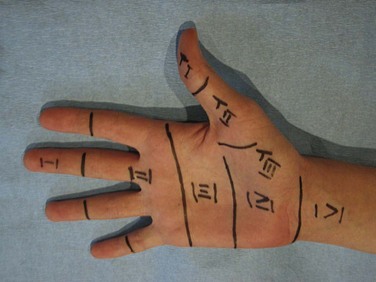
The arm and hand are divided into volar or palmar, and also dorsal, aspects. Distal to the elbow, structures are termed radial or ulnar to the middle finger axis rather than lateral and medial, respectively, because with forearm pronation and supination, the latter terms become confusing. The nomenclature of digits has become standardized. The hand has five digits, namely the thumb and four fingers (the thumb is not called a finger). The four fingers are respectively termed the index, long (middle), ring, and small (little) fingers. The use of numbers to designate digits is no longer accepted (Fig. 70-1). Within the hand, those structures close to the fingertips are termed distal, whereas those further up toward the wrist are termed proximal. Motion in a palmar direction is flexion, whereas dorsal motion is termed extension. Finger motion away from the long finger axis is termed abduction, whereas motion toward the axis of the long finger is termed adduction. The description of the motion of the thumb is sometimes confusing. Extension of the thumb is in the plane of the palm of the hand, whereas palmar abduction of the thumb is the motion that occurs at 90 degrees away from the plane of the palm. Finally, side to side motion of the wrist is termed radial and ulnar deviation.
Intrinsic muscles of the hand are those that have their origins and insertions in the hand, whereas the extrinsic muscles have their muscle bellies in the forearm and their tendon insertions in the hand. The intrinsic muscles that make up the thenar eminence are the abductor pollicis brevis (APB), flexor pollicis brevis (FPB), opponens pollicis (OP), and adductor pollicis (AP). There are four dorsal interossei that arise from adjacent sides of each metacarpal and provide abduction of the metacarpophalangeal (MP) joints of the index, middle, and ring fingers. There are three palmar interossei that adduct the index, ring, and little fingers toward the middle finger. Four lumbricals originate on the flexor digitorum profundus (FDP) tendons in the palm and insert on the radial sides of the extensor mechanisms of the four fingers. Together with the interossei, these bring about flexion of the MP joints and extension of the interphalangeal (IP) joints of the fingers (Fig. 70-2). The FPB flexes the thumb at the MP joint, in contrast with the extrinsic flexor pollicis longus (FPL), which flexes the thumb IP joint.
The hypothenar muscles consist of the flexor digiti minimi (FDM), which flexes the little finger at the MP joint, as well as the abductor digiti minimi (ADM) and opponens digiti minimi (ODM). A small muscle called the palmaris brevis is located transversally in the subcutaneous tissue at the base of the hypothenar imminence. It is innervated by the ulnar nerve, puckers the skin, and helps in cupping the skin of the palm during grip (Table 70-1).
Table 70-1 Intrinsic Muscles of the Hand
| MUSCLE | INNERVATION* | FUNCTION |
|---|---|---|
| Abductor pollicis brevis (APB) | Median | Abducts the thumb |
| Flexor pollicis brevis (FPB) | Median | Flexes the thumb |
| Opponens pollicis (OP) | Median | Opposes the thumb |
| Lumbricals | Median and ulnar | Flexes metacarpal phalangeal (MCP) joints and extends interphalangeal (IP) joints |
| Palmaris brevis | Ulnar | Wrinkles the skin on the medial (ulnar) side of the palm |
| Adductor pollicis (AdP) | Ulnar | Adducts the thumb |
| Abductor digiti minimi (ADM) | Ulnar | Abducts the small finger |
| Flexor digiti minimi (FDM) | Ulnar | Flexes the small digit |
| Opponens digiti minimi (ODM) | Ulnar | Opposes the small finger |
| Dorsal interossei | Ulnar | Abducts the fingers; flexes MCP joints and extends the IP joints |
| Palmar interossei | Ulnar | Adducts the fingers; flexes MCP joints and extends the IP joints |
* All the thenar intrinsic muscles are supplied by the median nerve except the AdP; all the remaining intrinsic muscles are supplied by the ulnar nerve except the two radial lumbricals.
The extrinsic muscles originate proximal to the wrist and comprise the long flexors and extensors of the wrist and digits. The extensors are located dorsally and are divided into three subgroups. The radialmost subgroup is termed the mobile wad and comprises the brachioradialis (BR), extensor carpi radialis longus (ECRL), and extensor carpi radialis brevis (ECRB). The ECRL and ECRB extend the wrist and deviate it radially. The second group is located in a more superficial layer and comprises three muscles—namely, the extensor carpi ulnaris (ECU), extensor digiti minimi-quinti (EDM-Q), and extensor digitorum communis (EDC). The ECU deviates the wrist in an ulnar direction and extends the wrist, whereas the EDM and EDC extend the MP joints of the fingers. The third and deeper subgroup comprises four muscles, three of which act on the thumb; the remaining muscle influences the index finger. The abductor pollicis longus (APL), extensor pollicis longus (EPL), and extensor pollicis brevis (EPB) provide function to the thumb, and the extensor indicis proprius (EIP) extends the MP joint to the index finger. Last of the deep muscles is the supinator, which is located proximally in the forearm (Table 70-2).
Table 70-2 Extrinsic Muscles of the Dorsal Forearm
| MUSCLE | INNERVATION* | FUNCTION |
|---|---|---|
| Extensor pollicis brevis (EPB) | Radial | Abducts the hand and extends the thumb at the proximal phalanx |
| Abductor pollicis longus (APL) | Radial | Abducts the hand and thumb |
| Extensor carpi radialis longus (ECRL) | Radial | Extends and radially deviates the hand |
| Extensor carpi radialis brevis (ECRB) | Radial | Extends and radially deviates the hand |
| Extensor pollicis longus (EPL) | Radial | Extends the distal phalanx of the thumb |
| Extensor digitorum communis (EDC) | Radial | Extends the fingers and the hand |
| Extensor indicis proprius (EIP) | Radial | Extends the index finger |
| Extensor digiti minimi/quinti (EDM/Q) | Radial | Extends the small finger |
| Extensor carpi ulnaris (ECU) | Radial | Extends and ulnarly deviates the wrist |
| Supinator | Radial | Supination |
| Brachioradialis | Radial | Flexes the forearm |
* All muscles of the dorsal forearm are innervated by the radial nerve and its respective branches.
The extensor tendons pass through six compartments deep to the extensor retinaculum at the dorsum of the wrist. From radial to ulnar side, these tendons and compartments are arranged as follows. The first compartment contains the APL and EPB, which also forms the radial boundary of the so-called anatomic snuffbox. The second compartment consists of the ECRL and ECRB, and the third compartment (which also forms the ulnar boundary of the anatomic snuffbox) contains the EPL. The EIP and EDC pass through the fourth compartment and the EDM through the fifth compartment, where they overlie the distal radioulnar joint. The sixth compartment contains the ECU (Fig. 70-3).
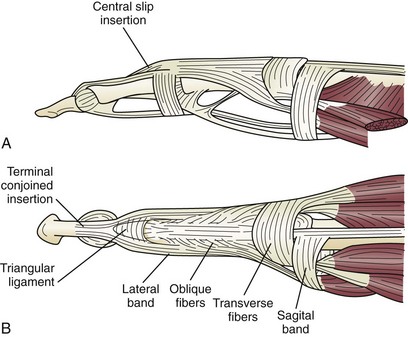
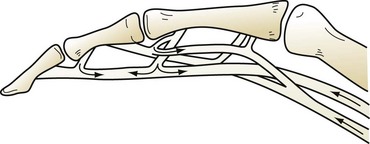
At the level of the MP joints, the long extrinsic extensor tendons broaden out to form the extensor hood. The proximal part of the hood at this level is called the sagittal band. It loops around the MP joint and blends into the volar plate, thus forming a lasso around the base of the proximal phalanx, through which it extends the MP joint. The insertions of the interossei and lumbricals enter into the extensor hood as the lateral bands. These lateral bands insert distally and dorsally to the axis of the PIP joint, and it is through this distal insertion that the intrinsic muscles (the interossei and lumbricals) are flexors of the MP joints and yet extensors of the IP joints. The extensor hood inserts to the base of the middle phalanx, which is termed the central slip, and finally proceeds on to the base of the distal phalanx, where it inserts through the terminal slip, thus extending the distal interphalangeal (DIP) joint (Fig. 70-4).
The extrinsic flexor muscles are located on the volar aspect of the forearm and are arranged in three layers. The superficial layer comprises four muscles—pronator teres (PT), flexor carpi radialis (FCR), flexor carpi ulnaris (FCU), and palmaris longus (PL). The PL muscle may be absent in as many as 10% to 12% of individuals. These muscles originate from the medial humeral epicondyle in the proximal forearm and function to flex the wrist and pronate the forearm. The intermediate layer consists of the flexor digitorum superficialis (FDS), which allows independent flexion of the proximal interphalangeal (PIP) joints of the fingers. In the deep layer, there are three muscles: the FPL, which flexes the IP joint to the thumb; the FDP, which flexes the DIP joints of the fingers; and a distal quadrangular muscle that spans between the radius and ulna termed the pronator quadratus, which helps in pronation of the forearm (Table 70-3).
Table 70-3 Extrinsic Muscles of the Volar Forearm
| MUSCLE | INNERVATION* | FUNCTION |
|---|---|---|
| Pronator teres (PT) | Median | Pronation |
| Flexor carpi radialis (FCR) | Median | Flexion and radial deviation of the wrist |
| Palmaris longus (PL) | Median | Flexion of the wrist |
| Flexor carpi ulnaris (FCU) | Ulnar | Flexion and ulnar deviation of the wrist |
| Flexor digitorum superficialis (FDS) | Median | Flexion of the proximal interphalangeal (PIP) joint |
| Flexor digitorum profundus (FDP) | Median and ulnar | Flexion of the distal interphalangeal (DIP) joint |
| Pronator quadratus | Median | Pronation |
| Flexor pollicis longus (FPL) | Median | Flexion of the thumb |
* All muscles of the volar forearm are innervated by the median nerve and its branches except the two ulnar digits of the FDP and FCU, which are innervated by the ulnar nerve.
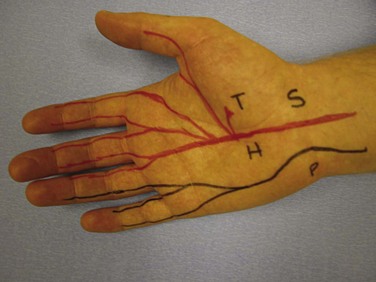
Nerve supply to the hand is by three nerves, the median, ulnar, and radial nerves. A knowledge of the surface anatomy of nerves helps when evaluating specific lacerating injuries (Fig. 70-5). The ulnar attachment to the flexor retinaculum is to the pisiform and hook of the hamate, and the radial attachment is to the scaphoid and ridge of the trapezium. The median nerve passes through the carpal tunnel between these landmarks. It gives sensation to the thumb, index finger, middle finger, and radial half of the ring finger. The palmar cutaneous branch of the median nerve originates from its radial side 5 to 6 cm proximal to the wrist, providing sensation to the palmar triangle. The ulnar nerve travels to the radial side of the pisiform and passes to the ulnar side of the hook of the hamate in its passage through Guyon’s canal. It gives sensation to the little finger and ulnar half of the ring finger; the dorsal branch of the ulnar nerve (arising proximal to the wrist and curving dorsally around the head of the ulna) supplies the same digits on their dorsal aspects. The superficial radial sensory nerve emerges from under the brachioradialis in the distal forearm, dividing into two or three branches proximal to the radial styloid, which then proceed in a subcutaneous course across the anatomic snuffbox, innervating the skin of the dorsum of the first web space. The number of fingers served by each nerve is variable. However, as an absolute rule, the palmar surfaces of the index and little fingers are always served by the median and ulnar nerves, respectively.
Examination And Diagnosis
Evaluation
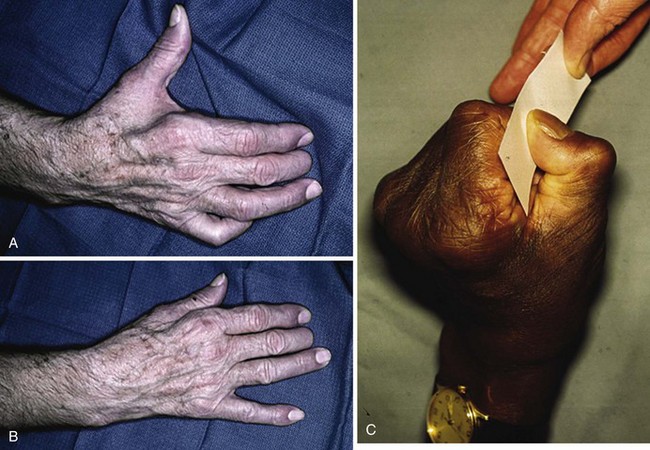
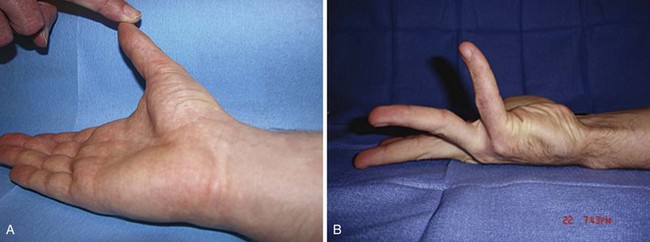
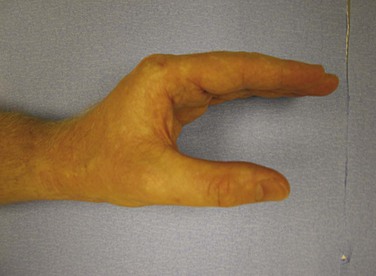
Basic instruments used in hand examination are shown in Figure 70-6. Examination of the resting posture of the hand can provide valuable information; for example, if a finger flexor tendon is severed, that affected finger does not assume its normal resting position in line with the natural flexion cascade of the adjacent digits (Fig. 70-7). Extensor tendon injuries may be indicated by a droop at the affected joint. A clawed posture of the little and ring fingers may be characteristic of an ulnar nerve injury (Fig. 70-8). Absence of sweating at the fingertips may imply a nerve injury in that particular distribution. Swelling and erythema may indicate a hand infection, and a purulent flexor tenosynovitis always results in a flexed posture of the digits. Rotational and angular digital deformities may occur when there are underlying fractures.
Neurovascular Examination
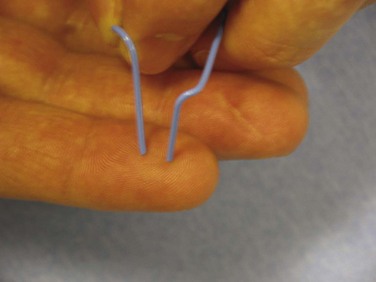
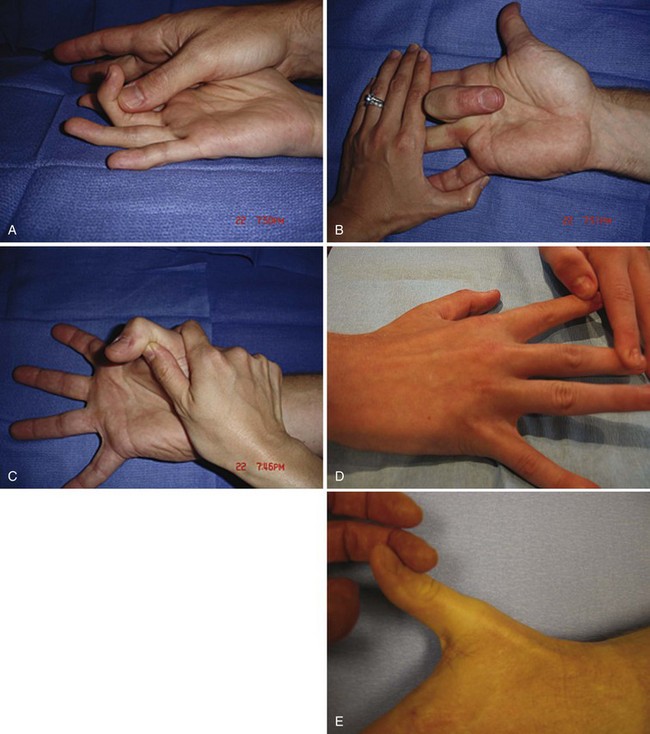
The Allen test confirms patency of the ulnar and radial arteries. Two-point sensory discrimination is the most sensitive method for testing for sensory loss and is easily done by using a bent paperclip (Fig. 70-9). The paperclip ends are set to a distance of approximately 5 mm apart for fingertip pulp sensory testing. The points are aligned along the axis of the finger. If this test is not reproducible because of an uncooperative patient, suspicion of a nerve injury can be confirmed by the tactile adherence test, in which a plastic pen is passed back and forth gently across the pulp on either side of each finger. Adhesion, because of the presence of sweat, is shown by slight but definite movement of the finger being examined (anesthetized finger pulp will not sweat).
There are two muscle tests that may provide the examiner with an absolute diagnosis of median or ulnar nerve injury. The motor function of the abductor pollicis brevis tests the median nerve. With the hand flat and facing palm up, the patient is asked to use his or her thumb to touch the examiner’s finger, which is held directly over the thenar eminence (Fig. 70-10). The FDM muscle function will test the motor supply of the ulnar nerve. In the same hand position, the patient raises her or his little finger vertically, flexing the MP joint to a 90-degree angle, with the IP joint held straight. Tests for radial nerve function and its branches require wrist extension, thumb extension, and finger extension at the MP joint.
Musculoskeletal Examination
The integrity of the tendons is individually tested (Fig. 70-11). Flexion at distal joints of the thumb and fingers confirms that the FPL and FDP, respectively, are intact. Testing of FDS tendons is more complex. It is not possible to flex the DIP joints independently of one another because of a common origin of the FDP tendons. Thus, the other fingers are fixed in extension by the examiner and the patient is asked to flex the remaining digits. Movement is produced by the FDS and occurs at the PIP joint. In approximately one third of patients, the FDS cannot produce little finger flexion. In 50% of these, in turn, there is a common origin with the ring finger, so flexion will occur if the ring finger is permitted to flex simultaneously. More uncommonly, there is no profundus tendon to the little finger and the superficialis inserts into the middle and distal phalanges. The long and short extensors (EPL and EPB) and long abductor of the thumb are tested by asking the patient to extend his or her thumb against resistance, whereas these tendons are individually palpated. Long extensors of the fingers are tested by asking the patient to extend them against resistance applied to the dorsum of the proximal phalanx.
Principles Of Treatment
In the case of injuries, treatment is directed at the specific structures damaged—skeletal, tendon, nerve, vessel, integument.1,2 In emergency situations, the goals of treatment are to maintain or restore distal circulation, obtain a healed wound, preserve motion, and retain distal sensation. Stable skeletal architecture is established in the primary phase of care because skeletal stability is essential for effective motion and function of the extremity. This also reestablishes skeletal length, straightens deformities, and corrects the compression or kinking of nerves and vessels. Arteries are also repaired in the acute phase of treatment to maintain distal tissue viability. Also, extrinsic compression on arteries must be released emergently, such as with compartment pressure problems. In clean-cut injuries, tendons can be repaired primarily. In situations in which there is a chance that tendon adhesions may form, such as when there are associated fractures, it is nonetheless better to repair tendons primarily with preservation of their length and, if necessary at a later date, to perform tenolysis. However, when there are open and contaminated wounds or a severe crushing injury, it is best to delay repair of tendon and nerve injuries.
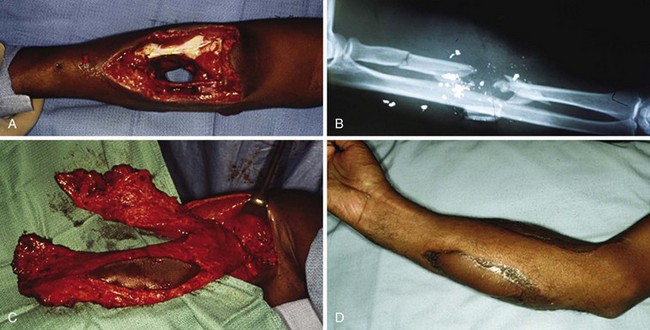
In severe soft tissue injuries, wound closure may not be possible immediately. Initial open treatment of the wound is directed to prevent an infection and protect critical deep structures by proper dressing and wound management (Fig. 70-12). Adequate débridement is essential, but appropriate soft tissue coverage must be achieved as soon as possible thereafter. The sooner the soft tissue coverage can be achieved, the less likely there will be a secondary deformity caused by fibrosis and joint contractures. The more rapidly hand therapy can be started, the better the chance for maximizing functional return. The treatment regimen must consist of débridement, rigid skeletal fixation, and early soft tissue resurfacing, possibly even requiring microvascular soft tissue reconstruction, followed by protected range-of-motion exercises as soon as possible. It has been shown that early soft tissue reconstruction results in improved function, decreased morbidity, and shortened hospital stay.
Anesthesia
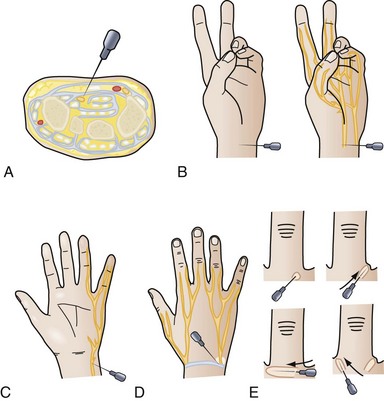
A digital block or median, ulnar, or radial wrist nerve block may be useful, especially for more limited emergency room procedures (Fig. 70-13). Digital nerve blocks usually do not include epinephrine, which could lead to vasospasm, but evidence has indicated the safety of distal blocks using an epinephrine solution. A maximum safe dose of lidocaine is 4 mg/kg.
Tourniquet Application
The tourniquet is used to provide a bloodless field so that clear visualization of all structures in the operative field is obtained. Penrose drains, rolled rubber glove fingers, or commercially available tourniquets can be used on digits. Great care must be taken when using any constrictive device on digits because narrow bands cause direct injury to underlying nerves and digital vessels. With the use of an arm tourniquet, the skin beneath the cuff must be protected with several wraps of cast padding. During skin preparation, this area must be kept dry to prevent blistering of the skin under an inflated cuff over moist padding. The cuff selected needs to be as wide as the diameter of the arm. Standard pressures used are 100 to 150 mm Hg higher than systolic blood pressure. The cuff is deflated every 2 hours for 15 to 20 minutes (5 minutes of reperfusion for every 30 minutes of tourniquet time) to revascularize distal tissues and relieve pressure on nerves locally before reinflating the cuff for more extensive procedures.3 Exsanguination of the extremities is performed by wrapping the extremity with a Martin’s bandage in all cases, except those involving infection or tumors. In these latter cases, because of the possibility of embolization by mechanical pressure, exsanguination by bandage wrapping needs to be avoided. Simple elevation of the extremity for a few minutes before tourniquet inflation suffices.1
Incisions
Incisions are of the Bruner zigzag or midaxial type, or combinations of these, to avoid longitudinal motion-restricting scars that cross palmar flexion creases (Fig. 70-14). The marginal edge of a skin graft with healthy skin is also a potential scar line, so the margin of the skin graft is designed to be in these same lines to prevent contractures across flexion creases. Palmar incisions follow the pattern of skin creases. Dorsal incisions on the fingers and wrist and incisions on the forearm may follow longitudinal straight lines.
Dressings and Splints
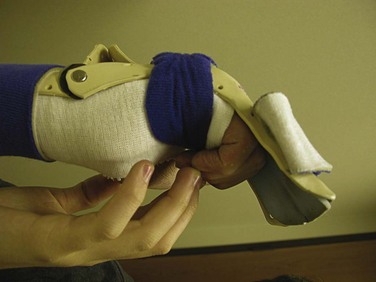
The purposes of dressings are to protect wounds, absorb drainage, and help splint repaired structures. The first layer consists of a nonadherent dressing and may contain an antibiotic. The next layer is soft and bulky and is usually followed by a firmer, more conforming external wrap. Conforming compression is useful, but constriction is harmful. Splints are made to protect only the part necessary to be immobilized and must not prevent motion in the remainder of the extremity. Often, patients keep the injured, operated, or infected hand in a flexed wrist position, which automatically causes the MP joints to extend, thereby placing the collateral ligaments in their shortest lengths. Edema fluid collects dorsally and the resulting dorsal hand swelling causes stiff joints. Thus, a splint that keeps the hand in the protected position extends the wrist 40 to 50 degrees, maintaining the MP joints at 70 degrees of flexion and the IP joints in a neutral position (Fig. 70-15). Postoperative hand elevation is essential to reduce edema.
Trauma
Lacerations, Fingertip, and Complex Soft Tissue Injuries
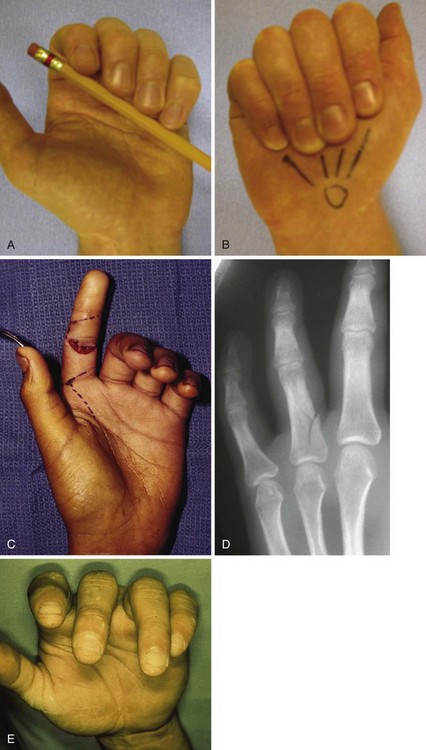
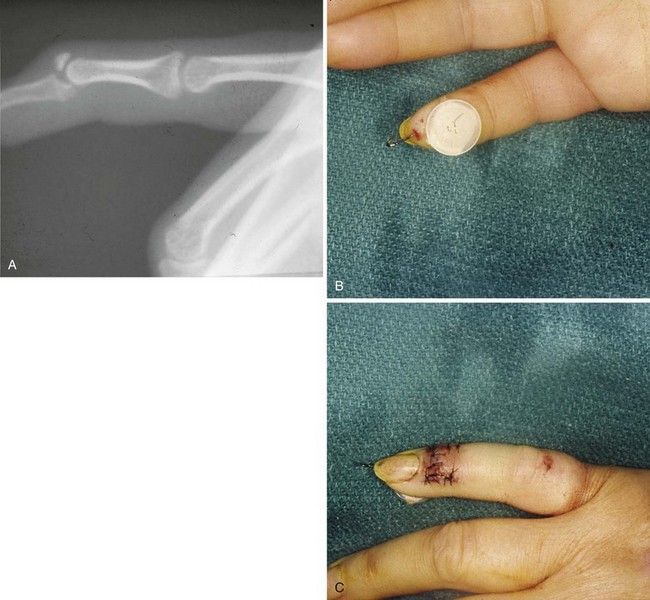
All patients who present with extremity injuries undergo radiography. Fractures of the distal phalanx are among the more commonly encountered hand fractures.4 A distal phalangeal fracture is appropriately splinted, reduced to improve alignment, or occasionally fixated internally if the fracture is unstable. Internal fixation is usually provided by simply placing a longitudinal 0.028-inch Kirschner wire. Appropriate antibiotics are administered because, technically, these are open fractures.
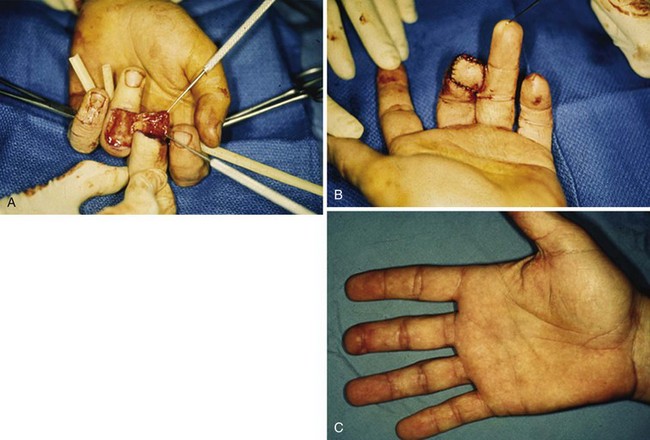
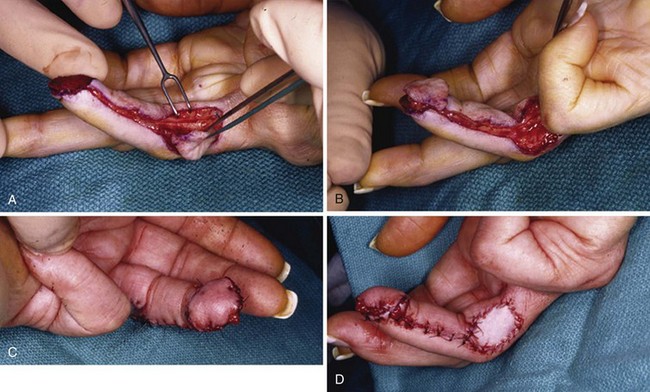
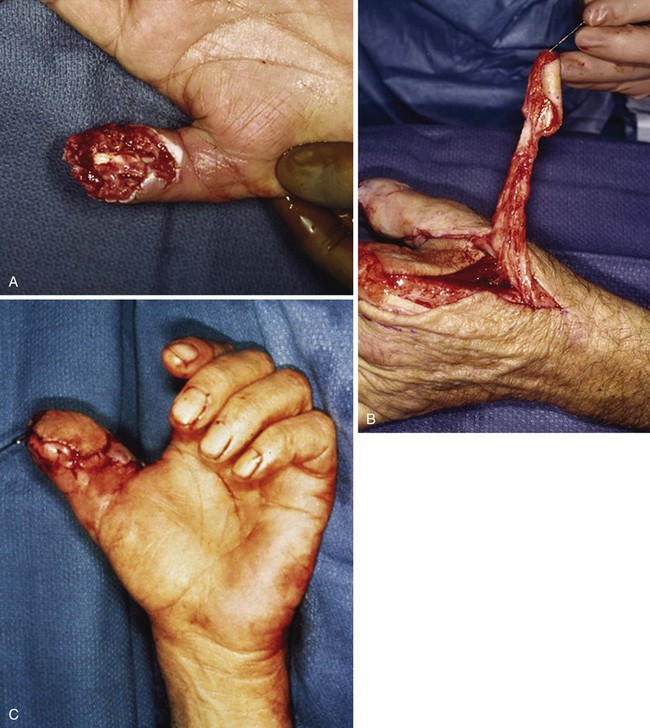
Volar fingertip injuries range from simple to more complex. Multiple digits may be involved, such as with lawnmower injuries. If bone is not exposed and a soft tissue defect of the finger pulp is smaller than 1 cm, the wound is best left open and managed with dressings. Such an injury will heal with excellent functional and cosmetic results. Larger soft tissue defects of the fingertip pulp are more appropriately treated with a small, full-thickness skin graft. However, if bone is exposed and the soft tissue wound is larger, flap coverage or revision of amputation by trimming back exposed bone to obtain soft tissue coverage should be considered. In a dorsally angulated fingertip amputation, soft tissue coverage can be achieved by a neurovascular V-Y advancement flap. If the soft tissue loss is angulated in a more volar direction, a cross-finger flap, adjacent finger digital island flap, or homodigital flap may be performed (Figs. 70-16 to 70-18).
Tendon Injuries
Flexor Tendons
Flexor tendon injuries usually result from lacerations or puncture wounds on the palmar surface of the hand, although flexor tendons can be avulsed from their distal bony insertions by sudden violent contractions. These are best treated by a surgeon experienced in the treatment of such injuries. Flexor tendon injuries are divided into five zones (Fig. 70-19). In zones 1, 2, and 4, each tendon is surrounded by a synovial sheath and contained within a semirigid fibro-osseous canal, either within the flexor tendon sheath of the digit or carpal tunnel. In the other zones, the flexor tendons are surrounded by loose areolar (paratenon) tissue. Those parts devoid of a fibrous sheath usually heal very well because of the good blood supply from the paratenon. Tendons in the carpal tunnel (zone 4) have their rich blood supply provided by the mesotenon; however, zones 1 and 2 have a precarious blood supply through the vincula; complementary nutritional support is provided by the synovial fluid in these latter two zones. For tendon gliding to occur, the mesotenon has disappeared in the digital flexor sheath except at the sites of the vincula that carry the vessels from the periosteum to the tendons (Fig. 70-20). Tendon zones to the thumb are T1 through T3.
After 4 weeks, a later secondary repair is generally not possible because of retraction of the musculotendinous unit so that reapproximation of the tendon ends produces undesirable joint flexion. In this situation, tendon graft repair may be required. The surgeon’s endeavors are directed at avoiding the four major complications that interfere with smooth gliding and the integrated action of tendons—adhesions, attenuation of the repair, repair rupture, and joint and soft tissue contractures. Prerequisites for tendon repair are aseptic conditions in the OR, with good lighting, good instruments, adequate anesthesia, and loupe magnification. A well-performed technical operation can be futile without proper postoperative hand therapy, splinting, and excellent patient compliance.5
Zone 2 flexor tendon injuries require special attention. This zone is also called Bunnell’s no man’s land. There are three tendons—the profundus and two slips of superficialis—that traverse zone 2 and they constantly interchange their mutual spatial relationships. Tendon injury in this region requires opening the existing laceration in the flexor tendon sheath by making a longitudinal trap door so that a flap of tendon sheath can be elevated. Care must be taken to avoid excising excessive portions of the flexor tendon sheath because bowstringing may result in ineffective finger flexion, although portions can be vented or excised to facilitate repair or prevent postoperative triggering. Total preservation of the A2 and A4 pulleys, previously thought to be essential, is no longer believed to be critical to success. One can excise up to 50% of the A2 and A4 pulleys without creating unnecessary tendon bowstringing if this is thought to be prudent to avoid the tendon repair impinging under the pulley.6 It has also been shown that one can incise the full length of the A4 pulley (but not excise it), without any biomechanical consequences.7 This is especially helpful when the zone 2 repair occurs proximate to the A4 pulley, the narrowest part of the flexor tendon sheath. Finally, wide awake anesthesia, which is local anesthetic infiltration using a solution of xylocaine with epinephrine, enables flexor tendon repair without the use of a tourniquet and ensures full patient cooperation during the procedure.8 This was previously thought to be unwise, but this has been proven to be unsubstantiated. Thus, one can determine intraoperatively that there is full flexor tendon excursion at the repair site without impingement under the pulleys as the patient flexes and extends his or her fingers before the skin incision is finally closed. All these novel and revolutionary concepts challenge previously accepted dogma with regard to zone 2 flexor tendon repairs and the significance of the various annular pulleys. It is often difficult to repair profundus and superficialis tendons if they are injured in zone 2. Nonetheless, both can be repaired because resection of the superficialis reduces overall grip strength, predisposes to a recurvatum and swan neck deformity at the PIP joint, and damages the vincula supply to the profundus.
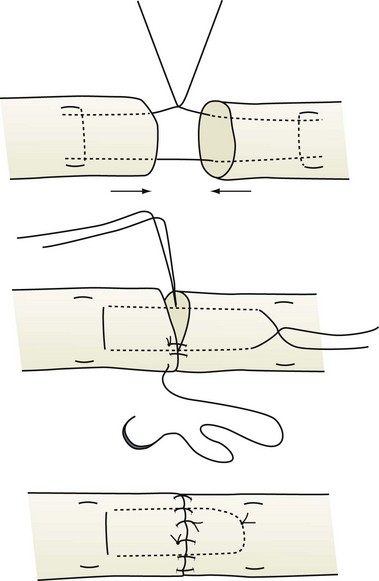
Usually, skin wounds have to be extended proximally and distally in a zigzag fashion to display the retracted divided tendon ends. Tendon ends are handled with a fine-toothed forceps and the tendon surface is never touched. The wrist is flexed and a small Keith needle is passed transversely through the proximal tendon, approximately 2 cm from the end, transfixing it to the skin and tendon sheath. In this way, immobilization of the tendon end facilitates a tension-free repair. Ragged tendon ends may be squared off sharply, but no more than 1 cm is resected or permanent finger contracture will result. The tendon ends are brought together by a single tension-holding, locking, core suture. Various locking core suture techniques have been described, but usually a modified Kessler-type suture is placed. A specifically placed locking loop increases the ultimate tensile strength of the tendon repair by 10% to 50% compared with a simple mattress suture. If this is not done, tension on the suture line can open up the repair, increasing the propensity for tendon gapping at the repair site. The ideal suture material for tendon repairs has not been found. A 4-0 coated polyester or braided nylon suture is the best material for the core suture. Increasing the number of suture strands that cross the tendon repair site and obtaining suture bites of at least 0.7 cm will increase the overall tensile strength of the actual repair.9 However, the more suture strands that are added, the greater will be the friction and edema within the flexor tendon sheath. A four- or six-stranded core repair appears to provide optimum repair strength and does not increases stiffness and friction at the repair site excessively. Some perform a four-stranded core repair by simply using a double-stranded type of suture material, whereas others place a second core suture with a single-stranded material. A four-stranded core repair permits a light, protected, composite grip for the duration of postoperative healing. Also, a running circumferential epitenon suture repair is also placed (Fig. 70-21). This not only helps smooth the repair but also adds to the ultimate tensile strength at the repair site and reduces gap formation. A peripheral 6-0 nylon suture serves this purpose.
The forces generated on FDP flexor tendons during passive finger flexion are 600 g and during active finger flexion are 2000 g; with strong active finger flexion, they are 8000 g. However, after tendon repair, the effects of wound healing, changes in elasticity, and added friction between the flexor tendons and their surrounding tissues will affect the overall work of flexion. There will be added frictional forces caused by edema, the presence of suture material, and the pulley system. The estimated work of flexion (resistance) increases by a factor of 50% after tendon repair. Thus, the estimated forces on repaired tendons, with 50% added for the work of flexion, are 900 g for passive finger flexion, 3000 g for active finger flexion, and 12,000 g for strong active flexion. The ultimate tensile strengths of various repairs are 2600 g for two-stranded and simple epitendinous repair, 4600 g for four-stranded and simple epitendinous repair, and 6800 g for six-stranded and simple epitendinous repair. The strength of the initial tendon repair decreases by approximately 25% during the first 3 weeks and then steadily increased thereafter to 6 weeks. Hence, if one is to undertake a postoperative active finger flexion protocol, then at least a four- or six-stranded core suture tendon repair is needed.10
Zone 1 flexor tendon injury may be caused by a penetrating injury. However, closed-traction injury may also cause profundus tendon avulsion, which most frequently involves the ring or middle finger. In the repair of a zone 1 injury, a pullout suture is necessary if the distal tendon length is insufficient to repair the tendon securely (Fig. 70-22), although suture bone anchors have facilitated this mode of tendon repair into bone at the base of the distal phalanx.
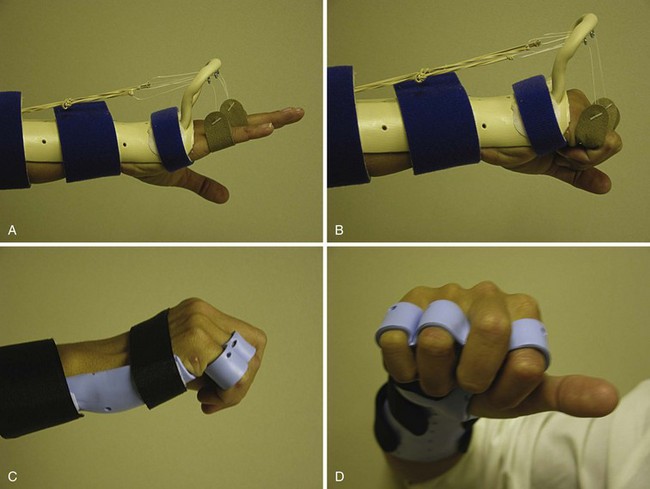
Postoperatively, hand elevation is important to reduce edema. The wrist is placed in approximately 20 degrees of flexion and the MP joint at approximately 60 to 70 degrees of flexion. The splint is molded against the fingers, with the IP joints fully extended. A system of rubber band dynamic traction may be used following the repair of flexor tendons in zone 2, with good results obtained in more than 80% of cases. Differential excursion between the two digital flexors is dramatically increased by a synergistic splint that allows for wrist extension and finger flexion. This position of wrist extension and MP joint flexion produces the least tension on a repaired flexor tendon during active digital flexion; thus, we have come to use the flexor hinge brace technique and the so-called place-and-hold protocol (Fig. 70-23). Of all the postoperative flexor tendon protocols, this enables the greatest overall tendon excursion of each of the FDS and FDP tendons and the most significant differential tendon gliding between the FDS and FDP repair sites, which theoretically would then reduce the risk of adhesion formation between the two tendons. A tenodesis brace with a wrist hinge is fabricated to allow for full wrist flexion, wrist extension of 30 degrees, and maintenance of MP joint flexion of at least 60 degrees. After composite passive digital flexion, the wrist is extended and passive finger flexion is maintained. The patient actively maintains digital flexion and holds that position for approximately 5 seconds. The patient is instructed to use the lightest muscle power necessary to maintain digital flexion. Wrist flexion and finger extension follow. This protected motion postoperative protocol is continued for 6 weeks.
Extensor Tendons
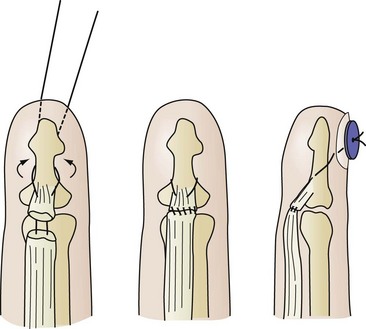
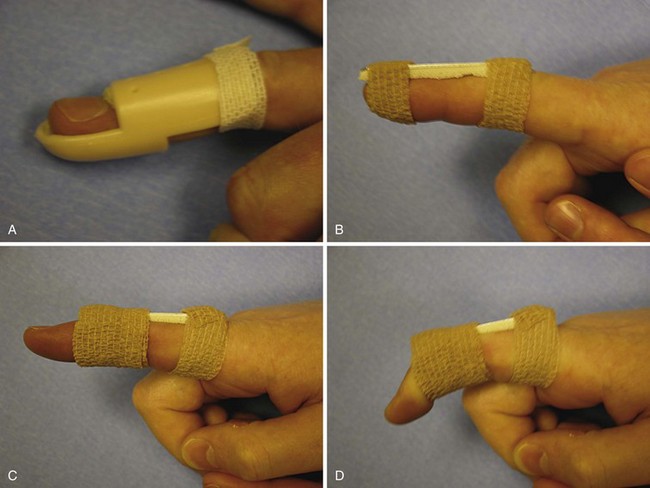
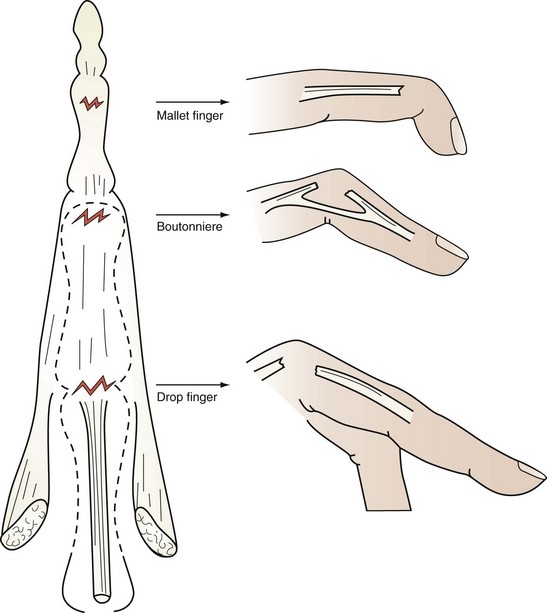
Extensor tendon avulsions are most likely to occur at the DIP joint from a jamming type of injury that results in a mallet finger deformity (Fig. 70-24). If a bone fragment representing 50% or more of the articular surface is involved, or if there is volar subluxation of the DIP joint, an open reduction with internal fixation is performed. If there is a tendon rupture only or a small piece of bone is avulsed with the tendon, good results can be obtained by 6 weeks of continuous splinting with the DIP joint in extension (Fig. 70-25). After this period of splinting, the DIP joint is further protected during sleep for 2 more weeks.
Closed tears through the triangular ligament may be caused by PIP joint subluxation or a jamming type of injury that results in a boutonniere deformity. The central slip attachment at the base of the middle phalanx is disrupted, so that extension of that joint is altered. The lateral bands lose their support dorsal to the PIP joint axis and slip volar and become flexors at the PIP joint and extensors of the DIP joint. The consequent deformity is one of flexion at the PIP joint and hyperextension at the DIP joint. Within 6 weeks of injury, these can be treated satisfactorily by extension splinting at the PIP joint, maintaining the DIP joint free for active flexion and extension (see Fig. 70-25). If there is an open laceration to the central slip mechanism and adjacent triangular ligament, direct suture repair or reinsertion into bone by means of bone anchor minisutures is performed, followed by the same postoperative protocol.
Extensor tendon injuries proximal to the PIP joint result in a drop finger (Fig. 70-26). These are repaired and splinted for 4 weeks. Common extensor tendon injuries over the dorsum of the hand and at the wrist must be repaired and then treated postoperatively by various different controlled motion protocols. One is a dynamic rubber band extension outrigger brace or use of a relative motion splint, in which the affected digit is kept at a more dorsal pitch to the adjacent fingers, thus relaxing the repaired tendon. This latter splint causes minimal interference with daily activities during rehabilitation (Fig. 70-27).11
Nerve Injuries
It has been suggested that optimal nerve regeneration and appropriate matching of axons in proximal and distal nerve segments result from a combination of paracrine-mediated neurotropism and contact guidance of sprouting proximal axons. Experimental evidence has suggested that the neurotropic chemical gradient can effectively guide regenerating axons at least 14 mm through a hollow nerve conduit in the rat model. The conduit allows diffusion of the neurotropic signal while preventing a mechanical fibrous block between the proximal and distal nerve segments. However, large-gap animal models (30 mm) have shown poor or no recovery using nerve conduits, suggesting that a finite limit exists for this technique. Although the gap length that can be bridged successfully in humans is still uncertain, many surgeons consider the use of bioresorbable nerve conduits for gap lengths up to 2 cm to be appropriate for small peripheral nerves. Nerve grafting remains the gold standard for large or mixed nerves and the brachial plexus. Appropriate conduits are polyglycolic acid tubes and semipermeable collagen tubes, which have shown similar experimental outcomes (Fig. 70-28).12
Nerve Transfers
If there may be a long distance between the site of nerve injury and distal muscle target, primary nerve repair may be fruitless, because muscle degeneration would have occurred by the time distal neural growth occurs. Muscle recovery is unlikely after an 18-month lapse. Thus, if nerve growth occurs at the rate of approximately 1 mm/day, a proximal motor nerve lesion more than 540 mm proximal to the hand will be doomed to failure. Hence, for proximal arm nerve and brachial plexus injuries, nerve transfers may result in a nerve repair that is closer to the muscle target. The donor nerve must be chosen so as to minimize morbidity from loss of the donor nerve. The donor nerve must be closely related to the denervated muscle so that the repair is performed much closer to the muscle target. Nerve transfers have revolutionized the repair of proximal nerve injuries so that distal muscle atrophy is minimized. For example, the classic Oberlin transfer uses part of the ulnar nerve (usually a single fascicle) for transfer to the musculocutaneous nerve and to the brachialis in the upper arm to restore elbow flexion.13 It is technically easy, quick, and effective. No significant motor or sensory deficits result in the territory of the ulnar nerve. This technique has become popular and is indicated for C5-6 brachial plexus lesions when C8-T1 is intact. It can also be used to neurotize a functioning free muscle transfer that may be required if the native muscles have already sustained atrophy because of prolonged denervation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree